 |
 |
- Search
| Korean J Fam Med > Volume 40(2); 2019 > Article |
|
Abstract
Background
Methods
Results
Figure. 1.
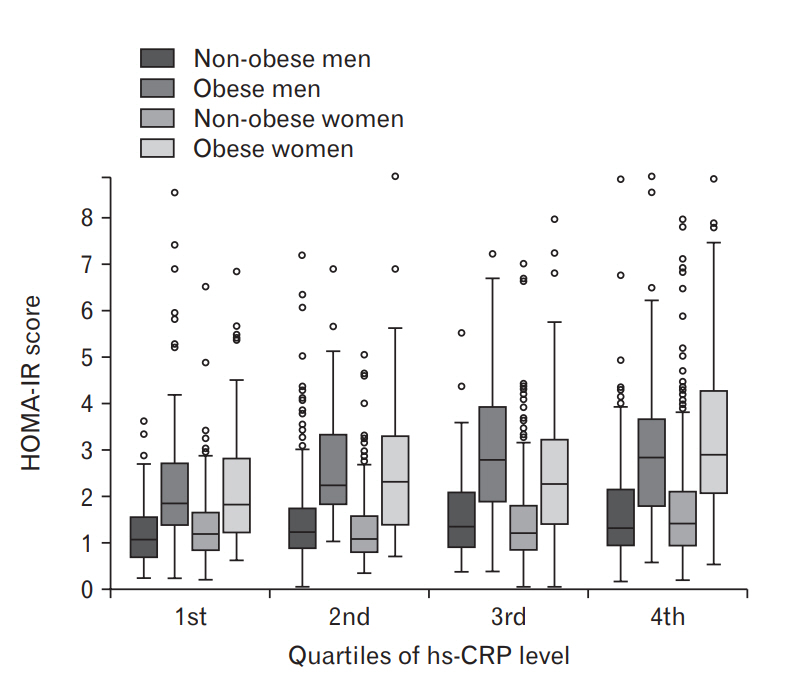
Table 1.
| Characteristic |
Men |
Women |
Total | ||
|---|---|---|---|---|---|
| Non-obese | Obese | Non-obese | Obese | ||
| Total | 740 (29.2) | 450 (17.9) | 1341 (40.4) | 482 (12.5) | 3013 (100.0) |
| Age (y) | 40.06±0.57 | 41.62±0.67 | 41.84±0.47 | 50.24±0.78 | 42.32±0.36 |
| Body mass index (kg/m2) | 22.35±0.08 | 27.74±0.13 | 21.39±0.07 | 27.75±0.14 | 23.60±0.09 |
| Height (cm) | 172.00±0.27 | 172.54±0.36 | 159.13±0.20 | 156.77±0.33 | 164.99±0.20 |
| Weight (kg) | 66.20±0.32 | 82.81±0.55 | 54.16±0.21 | 68.40±0.53 | 64.58±0.30 |
| Waist circumference (cm) | 80.65±0.25 | 93.33±0.38 | 73.91±0.23 | 88.80±0.41 | 81.22±0.23 |
| Systolic BP (mm Hg) | 115.85±0.61 | 122.24±0.73 | 109.81±0.45 | 120.64±0.95 | 115.15±0.38 |
| Diastolic BP (mm Hg) | 76.20±0.41 | 80.65±0.58 | 71.04±0.29 | 76.67±0.56 | 74.97±0.26 |
| Fasting glucose level (mg/dL) | 97.08±0.86 | 101.33±1.05 | 92.56±0.43 | 100.89±1.01 | 96.49±0.41 |
| TG level (mg/dL) | 135.97±4.95 | 213.64±9.68 | 94.48±1.66 | 144.00±3.99 | 134.12±2.64 |
| Total cholesterol level (mg/dL) | 186.98±1.37 | 199.85±1.78 | 187.64±0.93 | 198.55±1.88 | 190.99±0.69 |
| HDL level (mg/dL) | 50.88±0.49 | 44.25±0.50 | 57.86±0.42 | 49.61±0.59 | 52.36±0.27 |
| Serum insulin level (uIU/mL) | 6.27±0.19 | 10.97±0.28 | 6.30±0.12 | 10.91±0.39 | 7.70±0.11 |
| HOMA-IR score | 1.53±0.05 | 2.81±0.09 | 1.48±0.03 | 2.87±0.15 | 1.90±0.03 |
| High-sensitivity C-reactive protein level (mg/dL) | 0.81±0.04 | 1.12±0.07 | 0.70±0.03 | 1.46±0.10 | 0.90±0.03 |
| Smoking† | 244 (36.0) | 147 (36.3) | 52 (4.3) | 25 (6.2) | 468 (19.5) |
| Drinking‡ | 581 (80.4) | 334 (74.7) | 579 (46.1) | 199 (45.1) | 1693 (61.1) |
| Physical activity§ | 420 (57.7) | 249 (58.5) | 629 (50.0) | 207 (45.3) | 1505 (53.2) |
| Insulin resistance (HOMA-IR score ≥2.5) | 79 (10.8) | 193 (46.0) | 148 (9.6) | 214 (44.9) | 634 (20.9) |
| Abdominal obesity∥ | 49 (5.3) | 301 (66.6) | 100 (6.3) | 340 (69.2) | 790 (24.7) |
| Hypertriglyceridemia¶ | 204 (26.4) | 240 (54.0) | 203 (12.6) | 179 (35.8) | 826 (26.9) |
| Low HDL-cholesterol level** | 125 (15.8) | 140 (32.1) | 428 (27.9) | 277 (57.8) | 970 (28.8) |
| Metabolic syndrome | 103 (11.0) | 238 (50.0) | 159 (8.4) | 254 (48.8) | 754 (21.6) |
Values are presented as mean±standard error or unweighted number (weighted %).
BP, blood pressure; TG, triglyceride; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 2.
| Variable |
Non-obese |
Obese |
||
|---|---|---|---|---|
| Present | Absent | Present | Absent | |
| Men | ||||
| Abdominal obesity | 1.09 (0.54–1.65) | 0.79 (0.71–0.88) | 1.22 (1.02–1.41) | 0.93 (0.76–1.10) |
| Hypertriglyceridemia* | 0.94 (0.77–1.11) | 0.76 (0.66–0.86) | 1.13 (0.95–1.31) | 1.11 (0.90–1.31) |
| Low HDL-cholesterol level* | 1.07 (0.79–1.36) | 0.76 (0.67–0.85) | 1.32 (1.02–1.62) | 1.03 (0.88–1.17) |
| High fasting glucose level† | 0.91 (0.75–1.08) | 0.77 (0.67–0.87) | 1.12 (0.96–1.29) | 1.12 (0.91–1.32) |
| High BP | 0.76 (0.62–0.90) | 0.83 (0.72–0.94) | 1.27 (1.06–1.49) | 0.98 (0.81–1.15) |
| Metabolic syndrome† | 1.00 (0.73–1.27) | 0.79 (0.69–0.88) | 1.28 (1.07–1.49) | 0.96 (0.78–1.14) |
| Insulin resistance (HOMA-IR score ≥2.5)† | 1.06 (0.75–1.36) | 0.78 (0.69–0.87) | 1.36 (1.11–1.61) | 0.91 (0.79–1.04) |
| Women | ||||
| Abdominal obesity‡ | 0.81 (0.47–1.14) | 0.69 (0.63–0.76) | 1.67 (1.44–1.89) | 0.99 (0.72–1.27) |
| Hypertriglyceridemia | 0.80 (0.66–0.94) | 0.69 (0.62–0.76) | 1.51 (1.27–1.76) | 1.43 (1.17–1.69) |
| Low HDL-cholesterol level | 0.98 (0.82–1.15) | 0.59 (0.53–0.65) | 1.49 (1.25–1.74) | 1.41 (1.11–1.71) |
| High fasting glucose level | 0.94 (0.74–1.13) | 0.66 (0.59–0.73) | 1.51 (1.26–1.76) | 1.43 (1.16–1.69) |
| High BP† | 0.87 (0.68–1.05) | 0.67 (0.60–0.74) | 1.33 (1.09–1.56) | 1.56 (1.29–1.84) |
| Metabolic syndrome† | 1.07 (0.79–1.36) | 0.67 (0.60–0.73) | 1.49 (1.26–1.71) | 1.43 (1.11–1.75) |
| Insulin resistance (HOMA-IR score ≥2.5)* | 0.99 (0.74–1.24) | 0.67 (0.60–0.74) | 1.72 (1.45–2.00) | 1.24 (1.01–1.48) |
Values are presented as mean (95% confidence interval). Abdominal obesity, waist circumference >90 cm (men)/85 cm (women); hypertriglyceridemia, triglyceride level ≥150 mg/dL; low HDL-cholesterol, HDL level <40 mg/dL (men)/50 mg/dL (women); high fasting glucose level, fasting glucose level ≥100 mg/dL or use of antidiabetic agents or insulin; high BP, BP ≥130/85 mm Hg or intake of antihypertensive medications; metabolic syndrome, meeting 3 or more of the 5 aforementioned criteria.
HDL, high-density lipoprotein; BP, blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 3.
| Variable | P for trend | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile |
|---|---|---|---|---|---|
| Men, non-obese | |||||
| Quartiles of hs-CRP level | ≤0.30 | 0.30–0.50 | 0.50–0.80 | >0.80 | |
| Abdominal obesity | 0.89 | 1 | 1.54 (0.42–5.62) | 1.09 (0.40–2.97) | 1.38 (0.40–4.72) |
| Hypertriglyceridemia | 0.14 | 1 | 1.48 (0.86–2.53) | 1.85 (0.98–3.47) | 1.92 (1.05–3.50)* |
| Low HDL-cholesterol level | 0.09 | 1 | 1.61 (0.86–3.02) | 2.02 (1.06–3.83)* | 2.51 (1.20–5.22)* |
| High fasting glucose level | 0.10 | 1 | 1.79 (1.06–3.01)* | 1.78 (0.99–3.21) | 1.83 (0.96–3.46) |
| High BP | 0.10 | 1 | 0.72 (0.41–1.28) | 0.71 (0.40–1.26) | 0.72 (0.37–1.26) |
| Metabolic syndrome | 0.55 | 1 | 1.37 (0.63–2.99) | 1.70 (0.82–3.50) | 1.58 (0.58–4.27) |
| Men, obese | |||||
| Quartiles of hs-CRP level | ≤0.50 | 0.50–0.70 | 0.70–1.20 | >1.20 | |
| Abdominal obesity | 0.72 | 1 | 1.05 (0.49–2.28) | 0.75 (0.32–1.80) | 0.70 (0.28–1.76) |
| Hypertriglyceridemia | 0.52 | 1 | 1.49 (0.66–3.37) | 1.50 (0.73–3.07) | 1.07 (0.52–2.22) |
| Low HDL-cholesterol level | 0.33 | 1 | 0.50 (0.21–1.17) | 0.85 (0.39–1.83) | 0.64 (0.29–1.42) |
| High fasting glucose level | 0.32 | 1 | 0.56 (0.26–1.21) | 0.76 (0.39–1.49) | 1.01 (0.51–2.03) |
| High BP | 0.11 | 1 | 1.88 (0.89–3.99) | 2.04 (1.08–3.86)* | 2.24 (1.08–4.64)* |
| Metabolic syndrome | 0.32 | 1 | 0.77 (0.35–1.68) | 1.37 (0.67–2.80) | 1.07 (0.50–2.29) |
| Women, non-obese | |||||
| Quartiles of hs-CRP level | ≤0.30 | 0.30–0.40 | 0.40–0.70 | >0.70 | |
| Abdominal obesity | 0.45 | 1 | 0.58 (0.21–1.63) | 0.97 (0.34–2.79) | 0.57 (0.21–1.52) |
| Hypertriglyceridemia | <0.01 | 1 | 0.95 (0.52–1.72) | 1.27 (0.64–2.50) | 1.90 (1.08–3.35)* |
| Low HDL-cholesterol level | <0.01 | 1 | 1.76 (1.18–2.61)† | 1.52 (0.89–2.60) | 3.54 (2.29–5.48)‡ |
| High fasting glucose level | 0.12 | 1 | 0.78 (0.51–1.20) | 1.26 (0.71–2.24) | 1.25 (0.77–2.05) |
| High BP | 0.47 | 1 | 1.26 (0.74–2.16) | 0.84 (0.46–1.53) | 1.21 (0.66–2.21) |
| Metabolic syndrome | 0.01 | 1 | 1.12 (0.53–2.34) | 1.68 (0.75–3.77) | 2.63 (1.28–5.39)† |
| Women, obese | |||||
| Quartiles of hs-CRP level | ≤0.50 | 0.50–0.80 | 0.80–1.70 | >1.70 | |
| Abdominal obesity | 0.01 | 1 | 1.02 (0.37–2.77) | 1.69 (0.58–4.97) | 2.84 (1.16–6.96)* |
| Hypertriglyceridemia | 0.02 | 1 | 0.94 (0.32–2.72) | 1.79 (0.62–5.17) | 2.22 (0.87–5.69) |
| Low HDL-cholesterol level | 0.01 | 1 | 2.31 (0.79–6.81) | 5.07 (1.71–15.06)† | 3.21 (1.16–8.90)* |
| High fasting glucose level | 0.74 | 1 | 0.71 (0.27–1.86) | 0.62 (0.23–1.64) | 0.81 (0.32–2.05) |
| High BP | 0.57 | 1 | 0.83 (0.33–2.06) | 1.04 (0.36–3.03) | 0.65 (0.25–1.67) |
| Metabolic syndrome | 0.01 | 1 | 1.44 (0.54–3.86) | 3.87 (1.55–9.64)† | 2.57 (1.08–6.09)* |
Values are presented as hs-CRP quartile ranges (mg/dL) or odds ratio (95% confidence interval), unless otherwise stated. Adjusted for age, education, smoking, alcohol, body mass index, income, and physical activity. Abdominal obesity, waist circumference >90 cm (men)/85 cm (women); hypertriglyceridemia, TG level ≥150 mg/dL; low HDLcholesterol level, HDL-cholesterol level <40 mg/dL (men)/50 mg/dL (women); high fasting glucose, fasting glucose level ≥100 mg/dL or use of antidiabetic agents or insulin; high BP, blood pressure ≥130/85 mm Hg or use of antihypertensive medications; metabolic syndrome, meeting 3 or more of the 5 aforementioned criteria.
CRP, C-reactive protein; hs-CRP, high-sensitivity CRP; HDL, high-density lipoprotein; BP, blood pressure.
REFERENCES
-
METRICS

- Related articles in KJFM
-
Association between Obstructive Sleep Apnea and Glaucoma ;0(0)






