 |
 |
- Search
| Korean J Fam Med > Volume 43(3); 2022 > Article |
|
Abstract
Background
Evidence regarding the association between handgrip strength (HGS) and insulin resistance in a non-diabetic population is scarce. This study aimed to investigate the association between relative HGS and insulin resistance in older men without diabetes, using a representative sample of the Korean male population.
Methods
The study population comprised 206 participants aged 65–80 years, selected from the 2015 Korea National Health and Nutrition Examination Survey. Insulin resistance was defined as the upper tertile of the homeostatic model assessment of insulin resistance (HOMA-IR). Odds ratios and 95% confidence intervals for insulin resistance were assessed using multiple logistic regression analyses after adjusting for confounding variables.
Results
The prevalence of insulin resistance decreased with increasing relative HGS. The prevalence in the T1, T2, and T3 groups was 46.0%, 32.2%, and 26.4%, respectively. Compared with the individuals in the highest tertile of relative HGS, the odds ratio (95% confidence interval) for insulin resistance in individuals in the lowest quartile was 2.82 (1.10–7.21) after adjusting for age, smoking, alcohol consumption, aerobic exercise, resistance exercise, systolic blood pressure, total cholesterol, residential area, household income, and education level.
Conclusion
Lower relative HGS was inversely associated with an increased risk of insulin resistance in older Korean men without diabetes. In clinical practice, relative HGS, which is a simple and inexpensive tool, could be a useful measure for identifying older men with insulin resistance. Moreover, these findings suggest that muscle strengthening exercises should be considered to reduce insulin resistance and increase insulin sensitivity.
Insulin resistance, a condition characterized by the inability of insulin to increase glucose uptake and utilization, is an underlying cause of numerous medical disorders [1]. Individuals with insulin resistance are at a higher risk of developing type 2 diabetes mellitus [2], infectious disease [3], neurodegenerative disease [4], cerebrovascular disease [5], cardiovascular diseases [6], and certain cancers [7]. Furthermore, insulin resistance is associated with increased mortality risk [8]. Korea is rapidly becoming an aging society and is expected to be one of the most aged countries worldwide [9]. As the risk of developing insulin resistance increases with age, its risk is higher among the older population than in the general population [10,11].
A recent study showed that insulin resistance increased the risk of frailty and functional decline in older adults without diabetes [12]. In addition, insulin resistance was found to be associated with an increased risk of mortality among older adults without diabetes [13]. Therefore, early identification of insulin resistance and its prevention in non-diabetic older adults is important from a public health perspective. However, there are only a few standard tools for measuring insulin resistance. Although homeostasis model assessment of insulin resistance (HOMA-IR) is used to measure insulin resistance, it is not routinely quantified in clinical practice. Thus, a simple and accessible tool for predicting insulin resistance could be helpful for the early identification of older individuals with insulin resistance.
Handgrip strength (HGS) is a quick and simple clinical measure for assessing muscular strength. Moreover, HGS has been suggested as a simple screening tool for sarcopenia [14]. A recent study found that sarcopenia prevalence was higher in older men than in women in Korea [15]. Since HGS was associated with body mass index (BMI) [16], relative HGS, which is calculated as absolute HGS divided by BMI, has been widely used in recent studies [17-19].
Previous studies focusing on older individuals in Korea have revealed a significant association between HGS and osteoporosis, fracture, metabolic syndrome, cognitive impairment, and mortality [19-22]. Although a recent study has shown the relationship between HGS and insulin resistance in postmenopausal Korean women [23], few studies have examined the association between HGS and insulin resistance in a non-diabetic population. Thus, this study aimed to investigate the association between relative HGS and insulin resistance in older men without diabetes using a representative sample of the Korean male population.
This cross-sectional study used data from the 2015 Korea National Health and Nutrition Examination Survey (KNHANES). The KNHANES, which is conducted by the Korea Centers for Disease Control and Prevention (currently, Korea Disease Control and Prevention Agency), gathers nationally representative data on health indicators using complex survey designs to evaluate the health and nutritional status of South Koreans. The survey includes a health interview, health examination survey, and a nutritional survey. The target population of the survey is the civilian noninstitutionalized population of Korea. The sampling units comprise households systematically selected from a multistage stratification by age, sex distribution, and geographical area. Sampling weights representing the sample probabilities are assigned to each participant to ensure that the results are representative of the entire Korean population.
The KNHANES 2015 involved 7,380 participants, 673 of whom were men aged 65–80 years. Among them, we excluded participants who answered “yes” to the question, “Do you have a restriction of activity?” Among the remaining participants, those with a history of diabetes mellitus, arthritis, renal failure, liver cirrhosis, or cancer were excluded. We also excluded those who had not fasted for 8 hours prior to blood sampling and those whose fasting plasma glucose, insulin, BMI, and/or HGS data were missing. After exclusion, 206 participants were included in the final analysis.
All participants provided written informed consent prior to enrollment in the survey. The protocol for the KNHANES conducted in 2015 was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval no., 2015-01-02-6C). This study complied with the ethical principles of the Declaration of Helsinki.
In the 2015 KNHANES, participants were informed that their household had been randomly chosen to participate voluntarily in a nationwide representative survey performed by the Korea Centers for Disease Control and Prevention. They were also informed that they had the right to withdraw at any time based on the National Health Enhancement Act, supported by the National Statistics Law of Korea. Written informed consent was obtained from all citizens who agreed to participate.
Body weight and height were measured to the nearest 0.1kg and 0.1cm, respectively, using standardized techniques and equipment, and BMI was calculated using the following formula: weight/(height)2 (kg/m2). Blood pressure was measured in the right arm using a standard sphygmomanometer twice at 5-minute interval, and the average values were calculated. All measurements were performed by trained professionals. Blood samples were collected after overnight fasting. Fasting plasma glucose, insulin, total cholesterol, triglyceride, and high-density lipoprotein cholesterol levels were measured in a central certified laboratory.
HGS was measured using a digital grip strength dynamometer (Model TKK 5401; Takei Scientific Instruments Co. Ltd., Tokyo, Japan), with the participant in a standing position with the arm extended. The middle finger was held at 90° to the handle and measurements were taken while exhaling. The maximal hold time of the dynamometer was 3 seconds, and each hand was tested 3 times. The absolute HGS was calculated as the average of the three measurements of the dominant hand. Relative HGS was defined as the absolute HGS divided by BMI.
HOMA-IR was calculated using the following equation: fasting plasma glucose (mg/dL)×fasting insulin (mIU/mL)/405. In this study, insulin resistance was defined as the upper tertile of the HOMA-IR (>1.89).
Smoking was defined as current smoking and having smoked more than 100 cigarettes during their lifetime. Alcohol consumption was defined as alcohol consumption on at least 2 days per week. Aerobic exercise was defined as moderate-intensity activity for ≥2.5 hours per week or a combination of moderate- and high-intensity activities for ≥1 hour 15 minutes per week. Resistance exercise was defined as resistance exercise at least 3 times per week. Participants who performed resistance exercise were identified on the basis of their response to the following question: “How many days per week have you done resistance exercise, such as push-up, sit-up, dumbbell exercise, lift weights, the horizontal bar?”
Sampling weights were used to account for the complex design of the KNHANES survey. Therefore, we obtained valid estimates that represented the entire South Korean population to avoid bias. The participants were divided into three groups according to tertiles of relative HGS: T1, ≤1.387 kg/BMI; T2, 1.388–1.613 kg/BMI; and T3, ≥1.614 kg/ BMI. The characteristics of the study participants according to tertiles of relative HGS were determined through weighted one-way analysis of variance for continuous variables or weighted chi-square test for categorical variables. Pearson’s correlation analysis was used to identify the correlation between BMI and absolute and relative HGS. Odds ratios and 95% confidence intervals for insulin resistance were assessed using multiple logistic regression analyses after adjusting for confounding variables. All statistical analyses were conducted using IBM SPSS statistical software ver. 25.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P<0.05.
Among the 206 participants, 68, 69, and 69 were categorized to T1 (lowest), T2, and T3 (highest), respectively. Table 1 shows the characteristics of the study participants according to tertiles of relative HGS. Appendix 1 shows the results of the Pearson’s correlation analysis. While absolute HGS was positively associated with BMI (r=0.203, P=0.003), relative HGS was negatively associated with BMI (r=-0.438, P<0.001).
Figure 1 illustrates the prevalence of insulin resistance according to tertiles of relative HGS. The prevalence decreased with increasing relative HGS levels. Insulin resistance was prevalent in 46.0%, 32.2%, and 26.4% of participants in the T1, T2, and T3 groups, respectively.
Table 2 presents the odds ratio (95% confidence interval) of insulin resistance according to tertiles of relative HGS. Compared with the odds ratio for insulin resistance in individuals in the highest tertile, the odds ratio (95% confidence interval) in individuals in the lowest quartile was 2.82 (1.10–7.21) after adjusting for age, smoking, alcohol consumption, aerobic exercise, resistance exercise, systolic blood pressure, total cholesterol, residential area, household income, and education level.
This study investigated the relationship between relative HGS and insulin resistance in a representative sample of older Korean men without diabetes. In this cross-sectional study, we found that relative HGS was independently and inversely associated with insulin resistance in our study population after adjusting for potential confounding variables. Our findings are consistent with those of previous studies that reported an inverse association between HGS and insulin resistance [24,25] and that HGS can be a novel marker for assessing the risk of metabolic syndrome [20,26]. Moreover, our results suggest that relative HGS could be a useful marker for identifying older men with insulin resistance who do not have diabetes. Thus, our results expand on earlier findings regarding the association between HGS and insulin resistance.
In this study, absolute HGS was positively associated with BMI and relative HGS was negatively associated with BMI, which is also in agreement with the findings of previous studies [16,20,27]. Lifestyle behaviors, such as smoking, alcohol consumption, and exercise, influence insulin resistance [28-30]. Aerobic exercise and resistance exercise decrease insulin resistance independent of weight change [30]. Socioeconomic status also has an effect on insulin resistance. Several studies have shown that household income and education level are related to the risk of metabolic syndrome [31,32]. In this study, we had adjusted for these lifestyle behaviors and socioeconomic status in multiple logistic regression analyses to control for potential confounding factors.
Skeletal muscle plays a crucial role in glucose metabolism in the body and has a significant influence on insulin sensitivity. Skeletal muscle dysfunction is also involved in the development of insulin resistance. Skeletal muscles undergo several age-related changes that often lead to dysfunction. Thus, the increased incidence of insulin resistance in older adults may be closely associated with skeletal muscle aging [11]. Decreased expression of glucose transporter 4 (GLUT4) from a reduced muscle volume results in decreased insulin sensitivity in the aging skeletal muscle [33]. A previous study revealed that strength training improved insulin action in the skeletal muscle, independent of increase in muscle mass. In addition, strength training increased muscle GLUT4 content and the expression of several insulin-signaling proteins. Moreover, muscle biopsy in individuals with strength training showed increased protein content of GLUT4, protein kinase B-α/β, insulin receptor, and glycogen synthase as well as enhanced total activity of glycogen synthase [34].
This study had some limitations. First, the present study used a cross-sectional design, making it difficult to establish a causal relationship between relative HGS and insulin resistance in older men. Second, this study was confined to older Korean men; thus, the findings may have limited generalizability to other ethnic groups or women. Further studies are warranted to confirm the usefulness of relative HGS in evaluating the potential risk of insulin resistance in people of other ethnicities or women. Third, the mean values of BMI, which could influence the HOMA-IR levels, were significantly different between the tertiles of relative HGS. Further research is warranted to examine the association between relative HGS and insulin resistance among individuals with the same BMI. Lastly, we defined insulin resistance as the upper tertile of HOMA-IR. Although HOMA-IR is a generally accepted tool for quantifying insulin resistance in epidemiological studies, there are no definite cut-off values for defining insulin resistance based on HOMA-IR values. However, this definition of insulin resistance as the upper tertile of HOMA-IR is a commonly accepted approach and has been applied in previous studies [35,36]. Despite these potential limitations, to the best of our knowledge, this is the first population-based study to reveal the association between relative HGS and insulin resistance in older adults without diabetes. Moreover, this study used a nationally representative sample of older Korean men to improve the statistical validity of the findings.
In conclusion, lower relative HGS was inversely associated with an increased risk of insulin resistance in older Korean men without diabetes. Thus, relative HGS can be a useful measure for identifying older men with insulin resistance. Moreover, while longitudinal analyses are warranted to determine whether lower relative HGS is a risk factor for insulin resistance, the current findings suggest that muscle-strengthening exercises should nevertheless be considered for reducing insulin resistance and increasing insulin sensitivity.
Figure. 1.
Prevalence of insulin resistance according to the tertiles of relative handgrip strength.
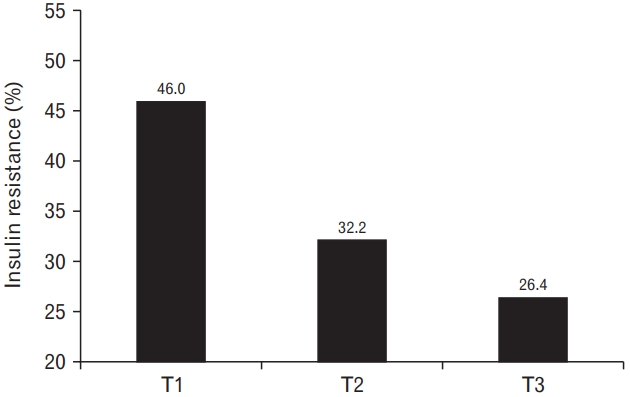
Table 1.
Participant characteristics by tertiles of relative handgrip strength
| Characteristic | T1 (lowest) | T2 | T3 (highest) | P-value | P-value* | P-value† | P-value‡ |
|---|---|---|---|---|---|---|---|
| Unweighted no. of participants | 68 | 69 | 69 | ||||
| Age (y) | 73.3±0.6 | 71.2±0.5 | 70.0±0.5 | <0.001 | 0.010 | <0.001 | 0.120 |
| Absolute handgrip strength (kg) | 29.9±0.9 | 36.3±0.4 | 41.2±0.6 | <0.001 | <0.001 | <0.001 | <0.001 |
| Body mass index (kg/m2) | 25.0±0.5 | 24.1±0.3 | 22.3±0.4 | 0.001 | 0.114 | <0.001 | <0.001 |
| Waist circumference (cm) | 89.4±1.5 | 86.9±0.7 | 82.8±1.1 | 0.002 | 0.122 | 0.001 | 0.001 |
| Systolic blood pressure (mm Hg) | 129.9±2.5 | 128.5±1.9 | 128.7±2.3 | 0.918 | 0.697 | 0.685 | 0.968 |
| Diastolic blood pressure (mm Hg) | 73.0±1.4 | 74.3±1.2 | 75.6±1.4 | 0.402 | 0.413 | 0.177 | 0.488 |
| Fasting plasma glucose (mg/dL) | 100.4±1.6 | 105.0±3.1 | 101.3±1.8 | 0.436 | 0.206 | 0.701 | 0.284 |
| Insulin (μIU/mL) | 10.9±2.6 | 8.2±2.0 | 6.4±0.4 | 0.174 | 0.413 | 0.093 | 0.371 |
| Homeostatic model assessment of insulin resistance | 2.8±0.7 | 2.4±0.7 | 1.6±0.1 | 0.177 | 0.644 | 0.107 | 0.321 |
| Total cholesterol (mg/dL) | 185.1±4.6 | 189.6±6.2 | 187.2±4.1 | 0.823 | 0.544 | 0.699 | 0.752 |
| Triglyceride (mg/dL) | 162.0±15.3 | 136.7±18.7 | 131.2±9.3 | 0.259 | 0.297 | 0.065 | 0.790 |
| High-density lipoprotein cholesterol (mg/dL) | 45.7±1.3 | 48.1±1.4 | 49.0±1.4 | 0.228 | 0.213 | 0.071 | 0.609 |
| Smoker (%) | 74.0±6.7 | 74.8±6.5 | 75.0±6.0 | 0.990 | 0.935 | 0.891 | 0.972 |
| Alcohol drinker (%) | 33.0±6.0 | 32.3±5.3 | 36.0±6.7 | 0.907 | 0.935 | 0.737 | 0.682 |
| Aerobic exerciser (%) | 55.2±5.9 | 45.0±6.0 | 44.5±6.1 | 0.356 | 0.218 | 0.198 | 0.957 |
| Resistance exerciser (%) | 38.0±6.6 | 30.1±6.7 | 34.3±5.5 | 0.659 | 0.362 | 0.672 | 0.636 |
| Residence in rural area (%) | 22.3±5.9 | 15.0±5.3 | 18.3±5.9 | 0.530 | 0.252 | 0.556 | 0.615 |
| Household income (US$/mo) | 1,988±222 | 2,739±352 | 2,555±355 | 0.046 | 0.023 | 0.173 | 0.702 |
| Education level (%) | 0.705 | 0.677 | 0.524 | 0.652 | |||
| ≤Elementary school | 36.8±7.0 | 44.3±6.9 | 34.8±6.9 | ||||
| Middle school | 16.2±4.2 | 15.7±5.0 | 16.2±4.3 | ||||
| High school | 35.1±7.1 | 26.0±5.5 | 27.9±7.2 | ||||
| ≥University | 11.9±4.1 | 14.0±3.8 | 21.0±5.6 |
Table 2.
Odds radios and 95% confidential intervals for insulin resistance (homeostatic model assessment of insulin resistance >66 percentile, 1.89) by tertiles of relative handgrip strength
Model 1: unadjusted; model 2: adjusted for age, smoking, alcohol consumption, aerobic exercise, and resistance exercise; and model 3: adjusted for age, smoking, alcohol consumption, aerobic exercise, resistance exercise, systolic blood pressure, total cholesterol, residential area, household income, and education level.
REFERENCES
1. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 2001;109 Suppl 2:S135-48.


3. Govender N, Khaliq OP, Moodley J, Naicker T. Insulin resistance in COVID-19 and diabetes. Prim Care Diabetes 2021;15:629-34.



4. Holscher C. Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs 2020;29:333-48.


5. Du XP, Xia J, Yang QD, Xu HW. Relationship between insulin resistance and clustering of risk factors of cerebrovascular disease. Hunan Yi Ke Da Xue Xue Bao 2000;25:163-5.

6. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab 2001;86:713-8.


7. Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, et al. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012;2012:789174.




8. Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care 2010;33:1179-85.




9. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323-35.



10. Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med 2000;30:327-46.

11. Shou J, Chen PJ, Xiao WH. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr 2020;12:14.




12. Rodriguez-Manas L, Angulo J, Carnicero JA, El Assar M, Garcia-Garcia FJ, Sinclair AJ. Dual effects of insulin resistance on mortality and function in non-diabetic older adults: findings from the Toledo Study of Healthy Aging. Geroscience 2021 Jun 1 [Epub]. https://doi.org/10.1007/s11357-021-00384-4


13. de Boer IH, Katz R, Chonchol MB, Fried LF, Ix JH, Kestenbaum B, et al. Insulin resistance, cystatin C, and mortality among older adults. Diabetes Care 2012;35:1355-60.




14. Blanquet M, Ducher G, Sauvage A, Dadet S, Guiyedi V, Farigon N, et al. Handgrip strength as a valid practical tool to screen early-onset sarcopenia in acute care wards: a first evaluation. Eur J Clin Nutr 2022;76:56-64.



15. Kim M, Won CW. Sarcopenia in Korean community-dwelling adults aged 70 years and older: application of screening and diagnostic tools from the Asian working group for sarcopenia 2019 update. J Am Med Dir Assoc 2020;21:752-8.


16. Hardy R, Cooper R, Aihie Sayer A, Ben-Shlomo Y, Cooper C, Deary IJ, et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PLoS One 2013;8:e56483.



17. Chon D, Shin J, Kim JH. Consideration of body mass index (BMI) in the association between hand grip strength and hypertension: Korean Longitudinal Study of Ageing (KLoSA). PLoS One 2020;15:e0241360.



18. Kim BM, Yi YH, Kim YJ, Lee SY, Lee JG, Cho YH, et al. Association between relative handgrip strength and dyslipidemia in Korean adults: findings of the 2014-2015 Korea National Health and Nutrition Examination Survey. Korean J Fam Med 2020;41:404-11.




19. Kim SW, Lee HA, Cho EH. Low handgrip strength is associated with low bone mineral density and fragility fractures in postmenopausal healthy Korean women. J Korean Med Sci 2012;27:744-7.




20. Yi DW, Khang AR, Lee HW, Son SM, Kang YH. Relative handgrip strength as a marker of metabolic syndrome: the Korea National Health and Nutrition Examination Survey (KNHANES) VI (2014-2015). Diabetes Metab Syndr Obes 2018;11:227-40.


21. Jang JY, Kim J. Association between handgrip strength and cognitive impairment in elderly Koreans: a population-based cross-sectional study. J Phys Ther Sci 2015;27:3911-5.



22. Bae EJ, Park NJ, Sohn HS, Kim YH. Handgrip strength and all-cause mortality in middle-aged and older Koreans. Int J Environ Res Public Health 2019;16:740.



23. Park HS, Lim JS, Lim SK. Determinants of bone mass and insulin resistance in Korean postmenopausal women: muscle area, strength, or composition? Yonsei Med J 2019;60:742-50.




24. Sayer AA, Syddall HE, Dennison EM, Martin HJ, Phillips DI, Cooper C, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. QJM 2007;100:707-13.



25. Lawman HG, Troiano RP, Perna FM, Wang CY, Fryar CD, Ogden CL. Associations of relative handgrip strength and cardiovascular disease biomarkers in U.S. adults, 2011-2012. Am J Prev Med 2016;50:677-83.



26. Yang EJ, Lim S, Lim JY, Kim KW, Jang HC, Paik NJ. Association between muscle strength and metabolic syndrome in older Korean men and women: the Korean Longitudinal Study on Health and Aging. Metabolism 2012;61:317-24.


27. Lee WJ, Peng LN, Chiou ST, Chen LK. Relative handgrip strength is a simple indicator of cardiometabolic risk among middle-aged and older people: a nationwide population-based study in Taiwan. PLoS One 2016;11:e0160876.



28. Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance: a potential link with the insulin resistance syndrome. J Intern Med 1993;233:327-32.


29. Bell RA, Mayer-Davis EJ, Martin MA, D’Agostino RB Jr, Haffner SM. Associations between alcohol consumption and insulin sensitivity and cardiovascular disease risk factors: the Insulin Resistance and Atherosclerosis Study. Diabetes Care 2000;23:1630-6.



30. Bell LM, Watts K, Siafarikas A, Thompson A, Ratnam N, Bulsara M, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab 2007;92:4230-5.



31. Zhan Y, Yu J, Chen R, Gao J, Ding R, Fu Y, et al. Socioeconomic status and metabolic syndrome in the general population of China: a cross-sectional study. BMC Public Health 2012;12:921.




32. Park SJ, Kang HT, Nam CM, Park BJ, Linton JA, Lee YJ. Sex differences in the relationship between socioeconomic status and metabolic syndrome: the Korean National Health and Nutrition Examination Survey. Diabetes Res Clin Pract 2012;96:400-6.


33. Tsao TS, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes 1996;45:28-36.


34. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004;53:294-305.



- TOOLS







