 |
 |
- Search
| Korean J Fam Med > Epub ahead of print |
|
Abstract
Background
Methods
Results
Figure.┬Ā1.
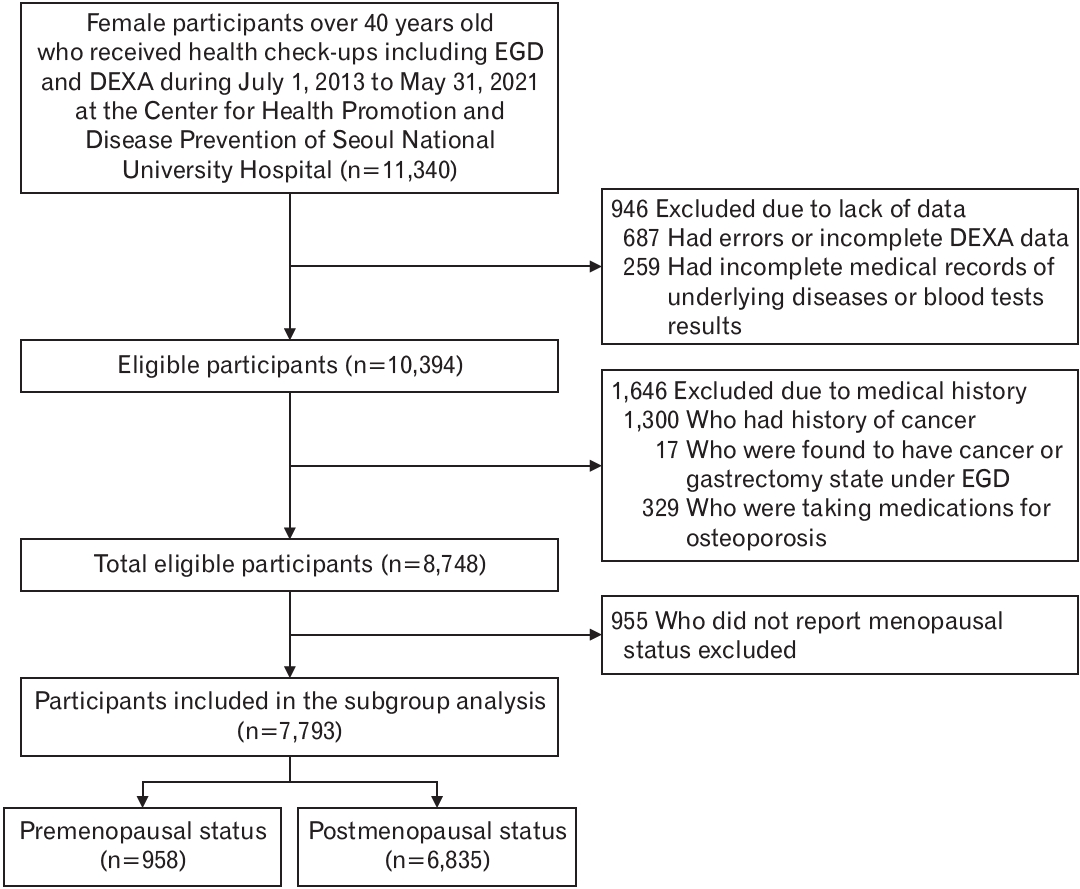
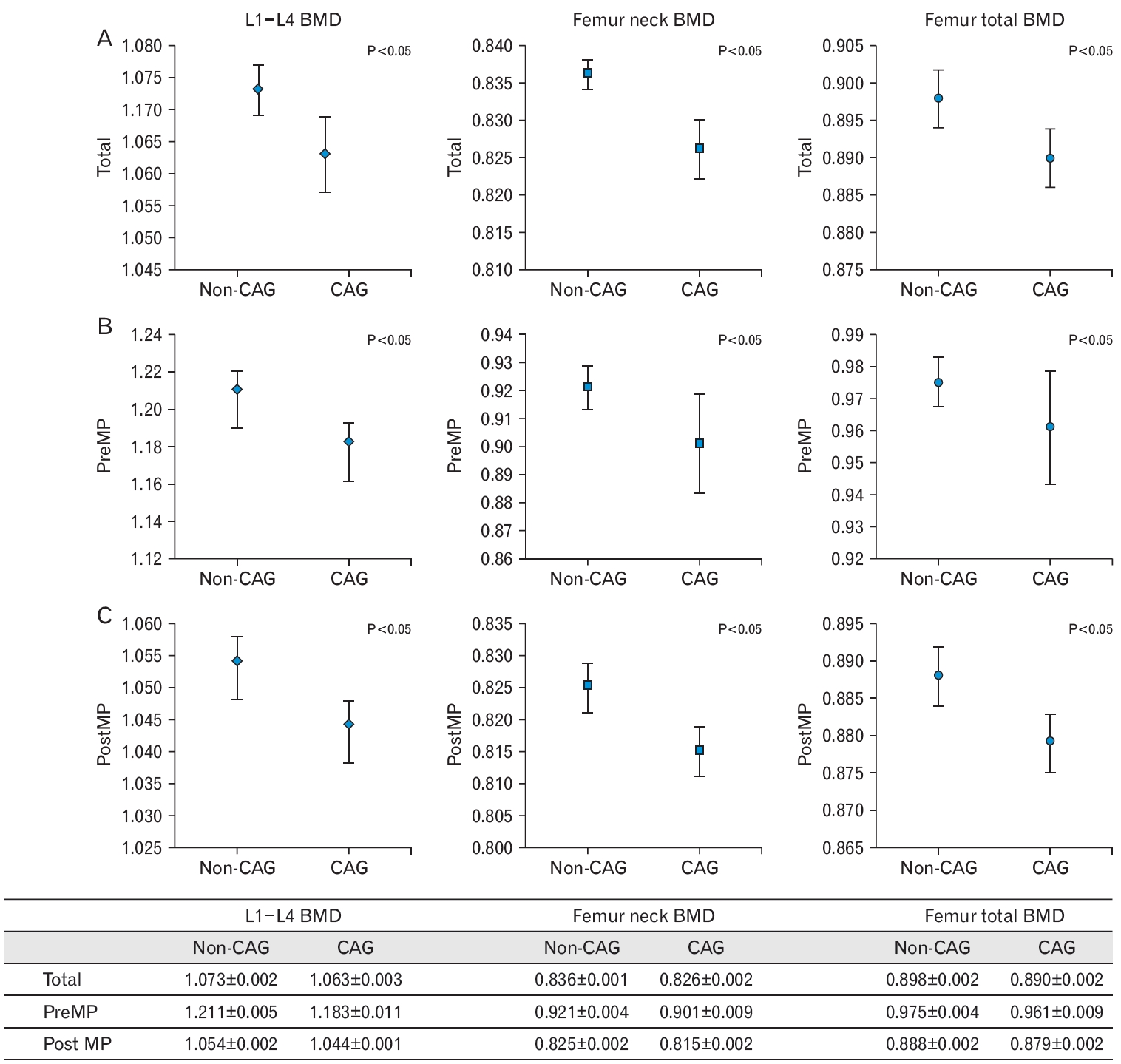
Figure.┬Ā2.
Table┬Ā1.
| Characteristic | Total (n=8,748) | CAG (n=3,076) | Non-CAG (n=5,672) | P-value |
|---|---|---|---|---|
| Age (y) | 59.61┬▒8.03 | 62.63┬▒7.89 | 57.97┬▒7.61 | <0.001 |
| Body mass index (kg/m2) | 23.07┬▒3.13 | 23.25┬▒3.10 | 22.97┬▒3.14 | <0.001 |
| Waist circumference (cm) | 81.59┬▒8.65 | 82.40┬▒8.53 | 81.15┬▒8.68 | <0.001 |
| Hypertension* | 3,038 (34.73) | 1,209 (39.30) | 1,829 (32.25) | <0.001 |
| DyslipidemiaŌĆĀ | 4,418 (50.50) | 1,630 (52.99) | 2,788 (49.15) | 0.001 |
| Diabetes mellitusŌĆĪ | 1,435 (16.40) | 586 (19.05) | 849 (14.97) | <0.001 |
| Chronic kidney disease┬¦ | 441 (5.04) | 185 (6.01) | 256 (4.51) | 0.002 |
| Thyroid diseaseŌłź | 1,427 (16.31) | 539 (17.52) | 888 (15.66) | 0.024 |
| Current smoking┬Č | 215 (2.46) | 51 (1.66) | 164 (2.89) | <0.001 |
| Current drinking# | 122 (1.39) | 52 (1.69) | 70 (1.23) | 0.025 |
| Moderate to severe physical activity** | 545 (6.23) | 185 (6.01) | 360 (6.35) | 0.825 |
| MenopauseŌĆĀŌĆĀ | 6,835 (78.13) | 2,524 (82.05) | 4,311 (84.46) | <0.001 |
| Hormone replace therapyŌĆĪŌĆĪ | 1,282 (16.43) | 488 (18.03) | 794 (15.58) | <0.001 |
| L1ŌĆōL4 BMD (g/cm2) | 1.070┬▒0.160 | 1.044┬▒0.157 | 1.084┬▒0.160 | <0.001 |
| Femur neck BMD (g/cm2) | 0.833┬▒0.116 | 0.810┬▒0.111 | 0.845┬▒0.118 | <0.001 |
| Femur total BMD (g/cm2) | 0.896┬▒0.123 | 0.876┬▒0.120 | 0.906┬▒0.124 | <0.001 |
| Low BMD┬¦┬¦ | 4,164 (47.60) | 1,747 (56.79) | 2,417 (42.61) | <0.001 |
Values are presented as mean┬▒standard deviation or number (%). P-values were calculated using the independent t-test for continuous outcomes and Žć2-test for discrete outcomes.
CAG, chronic atrophic gastritis; BMD, bone mineral density; CKD, chronic kidney disease; TSH, thyroid-stimulating hormone.
* Those who had a history of hypertension or taking hypertensive medications or systolic blood pressure Ōēź140 mm Hg.
ŌĆĀ Those who had history of dyslipidemia or taking medications or total cholesterol Ōēź240 mg/dL or low-density lipoprotein Ōēź160 mg/dL or triglyceride Ōēź200 mg/dL or high-density lipoprotein <40 mg/dL.
ŌĆĪ Those who had a history of diabetes mellitus or taking diabetes medications or glycated hemoglobin Ōēź6.5% or fasting blood sugar Ōēź126 mg/dL.
┬¦ Those who had a history of CKD or taking medications due to CKD or calculated glomerular filtration rate using the Modification of Diet in Renal Diseases equation <60 mL/min.
Ōłź Those who had a history of hypothyroidism or hyperthyroidism or taking medications for thyroid dysfunction or TSH <0.35 mIU/L or TSH >4.94 mIU/L.
Table┬Ā2.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Total | ||
| ŌĆāModel 1* | 1.77 (1.62ŌĆō1.93) | <0.001 |
| ŌĆāModel 2ŌĆĀ | 1.25 (1.13ŌĆō1.37) | <0.001 |
| ŌĆāModel 3ŌĆĪ | 1.26 (1.13ŌĆō1.40) | <0.001 |
| Premenopausal women | ||
| ŌĆāModel 1* | 1.01 (0.64ŌĆō1.57) | 0.976 |
| ŌĆāModel 2ŌĆĀ | 1.07 (0.68ŌĆō1.70) | 0.765 |
| ŌĆāModel 3┬¦ | 1.14 (0.71ŌĆō1.84) | 0.586 |
| Postmenopausal women | ||
| ŌĆāModel 1* | 1.60 (1.45ŌĆō1.77) | <0.001 |
| ŌĆāModel 2ŌĆĀ | 1.28 (1.15ŌĆō1.42) | <0.001 |
| ŌĆāModel 3Ōłź | 1.27 (1.14ŌĆō1.41) | <0.001 |
Odds ratios were calculated by multivariate logistic regression.
CI, confidence interval; BMI, body mass index; WC, waist circumference; DM, diabetes mellitus; CKD, chronic kidney disease.
ŌĆĪ Adjusted for age, BMI, WC, hypertension, dyslipidemia, DM, CKD, thyroid disease, smoking status, alcohol consumption, physical activity, menopausal status, and hormone replacement therapy.
REFERENCES
-
METRICS

-
- 0 Crossref
- 398 View
- 15 Download
- Related articles in KJFM
-
Association between Near Work Time and Depression among Workers in South Korea2021 September;42(5)
Association between Abdominal Obesity and Oxidative Stress in Korean Adults2019 November;40(6)






