Effect of Alcohol Consumption on Risk of Hyperhomocysteinemia Based on Alcohol-Related Facial Flushing Response
Article information
Abstract
Background
This study examined the relationship between alcohol consumption and hyperhomocysteinemia based on facial flushing caused by drinking.
Methods
Among male patients aged ≥ 18 years who visited Health Promotion Center of Chungnam National University Hospital in Daejeon from January 2008 to December 2010, 948 males (182 nondrinkers, 348 subjects with drinking-related facial flushing, and 418 subjects without drinking-related facial flushing) were selected. After adjusting for confounding factors such as age, body mass index, hypertension, diabetes, smoking, triglycerides, high density lipoprotein cholesterol, and gamma-glutamyl transpeptidase, a multiple logistic regression analysis was performed to assess the risk of hyperhomocysteinemia in the nonfacial flushing and facial flushing groups compared with the nondrinkers.
Results
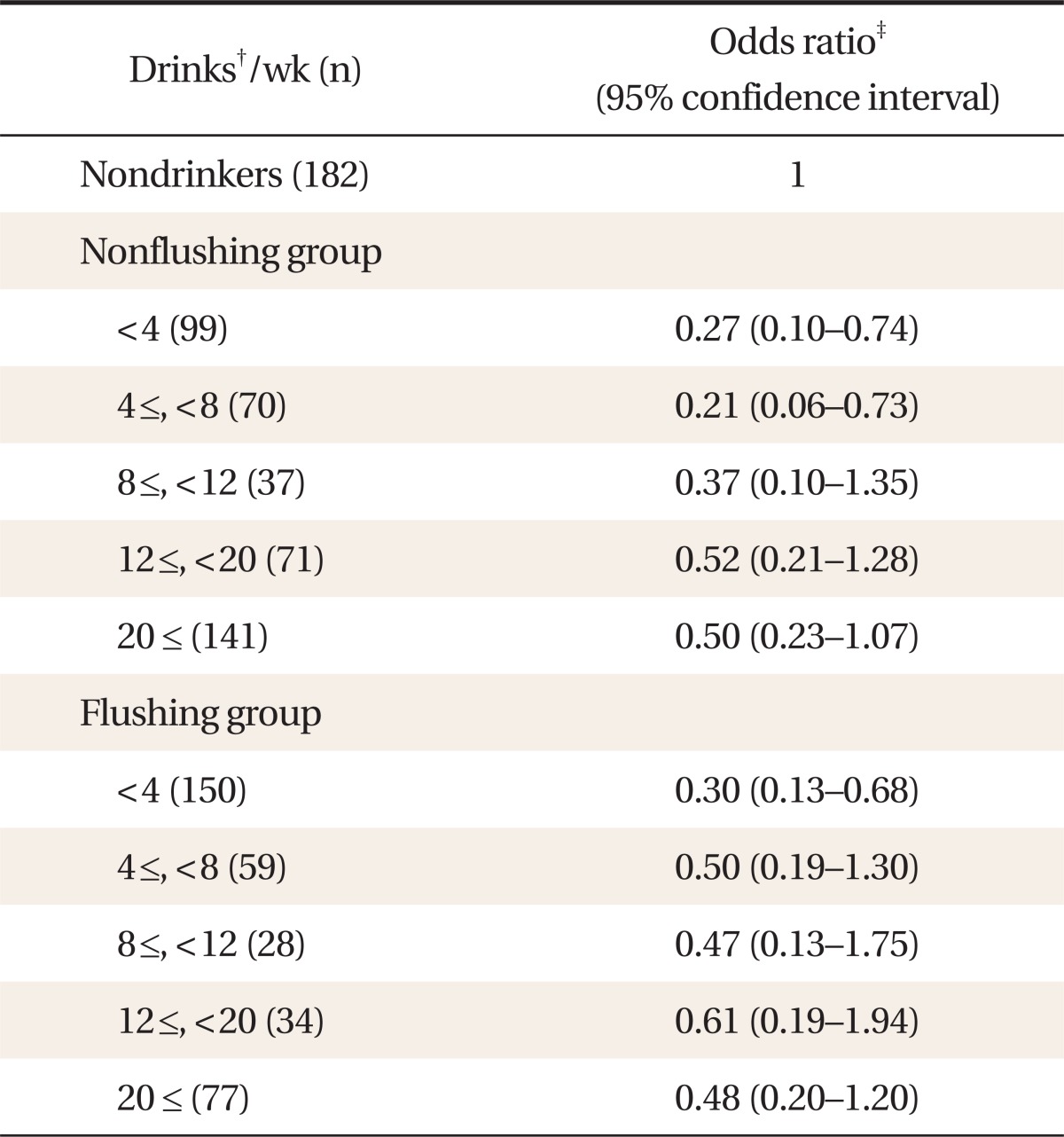
After adjusting for confounding factors, risk of hyperhomocysteinemia was significantly lower in the group with a weekly alcohol consumption of < 8 standard drinks (1 drink = 14 g alcohol) in the nonfacial flushing group (<4 drinks: odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10 to 0.74; 4≤, <8 drinks: OR, 0.21; 95% CI, 0.06 to 0.73). Risk of hyperhomocysteinemia was significantly lower in the group with a weekly alcohol consumption < 4 drinks in the facial flushing group (OR, 0.30; 95% CI, 0.13 to 0.68).
Conclusion
Our results suggest that the risk of hyperhomocysteinemia is likely lowered by alcohol consumption based on drinking quantity, as lowering the risk of hyperhomocysteinemia differs depending on vulnerability associated with facial flushing.
INTRODUCTION
Facial flushing is a common symptom of alcohol consumption in Koreans. The exact biochemical mechanism of drinkingrelated facial flushing is unknown, but the inactive aldehyde dehydrogenase (ALDH) 2 enzyme is involved, promoting alcohol intolerance.1,2) Higuchi et al.3) stated that facial flushing triggers unpleasant emotions in the drinker, reducing drinking frequency and quantity. They reported that the risk for alcohol abuse by men was approximately 3.0 times higher among atypical (occasional) flushers after drinking and 1.7 times higher among nonflushers (never) than among typical (always) flushers. This finding is consistent with a study that reveals that inactive ALDH is associated with a reduction in drinking amount and frequency.4)
Hyperhomocysteinemia has been reported as an independent risk factor for coronary artery disease and arteriosclerosis, along with diabetes, hyperlipidemia, smoking, and hypertension,5) and is regarded as causing a wide range of vascular diseases, such as coronary artery disease, cerebrovascular disease, peripheral vascular disease, and phlebothrombosis.6)
Homocysteine is produced during protein decomposition in ingested food. Hyperhomocysteinemia is attributed to common genetic and acquired factors, including folate and vitamin B12 deficiencies.7) Additionally, age, sex, menopause, nutritional condition, chronic renal failure, cancer, smoking, and drugs are associated with elevated homocysteine levels.8)
Regarding the relationship between alcohol consumption and homocysteine levels, Cravo et al.9) documented that excessive drinking led to an increase in blood homocysteine concentrations. Conversely, Ubbink et al.10) demonstrated that moderate drinking reduced blood homocysteine concentration. Additionally, a study targeting United States women found no significant relationship between drinking and homocysteine levels.11)
Therefore, the relationship between drinking and hyperhomocysteinemia is not understood fully. Reactions to the same amount of alcohol can differ between individuals and among ethnic groups. Also, there are only a few studies on the relationship between drinking and hyperhomocysteinemia in Koreans. Furthermore, the above mentioned studies did not consider alcohol-related facial flushing. Therefore, this study explored the relationship between alcohol consumption and hyperhomocysteinemia in Koreans, as a large proportion of them experience alcohol-related facial flushing.
METHODS
1. Study Subjects
The study targeted Korean adult males who visited Chungnam National University Hospital in Daejeon, Korea, from January 2008 to December 2010 for health examinations. The authors excluded patients with conditions that would likely have affected homocysteine concentrations, such as chronic renal failure, hypothyroidism, and malignant diseases. Additionally, drug users, including those taking methotrexate, phenytoin, carbamazepine, theophylline, niacin, colestipol, multivitamins, and other dietary supplements, were excluded from this study. In total, there were 948 subjects: 182 nondrinkers, 348 drinkers with alcohol-related facial flushing, and 418 drinkers with no facial flushing. The institutional review board of Chungnam National University Hospital approved this study (IRB number: CNUH 2012-08-020-002).
2. Study Methods
This retrospective study reviewed the medical records of the subjects from their health examination. Data included the subjects' age, smoking status, and history acquired through self-administered questionnaires. It also included anthropometric data on height and weight. Body mass index (BMI) was calculated from the measured height and weight using the Quetelet Index.12)
To identify drinking-related characteristics, typical drinking quantity per drinking day and drinking frequency per week over the previous year were investigated. The amount consumed per week was calculated by multiplying the weekly drinking frequency and drinking amount per drinking day. In terms of the amount consumed, 14 g of alcohol was considered a standard drink in accordance with the guidelines suggested by the National Institute on Alcohol Abuse and Alcoholism.13) In response to a questionnaire, drinking-related facial flushing was classified as always, sometimes, and never. Subjects who responded always and sometimes were classified as part of the facial flushing group, while those responding never were classified as part of the nonfacial flushing group. This classification was based on a study by Yokoyama et al.,14) who found that the sensitivity and specificity of this classification for identifying inactive ALDH 2 were 96.1% and 79.0%, respectively.
The blood levels of triglyceride, total cholesterol, high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transpeptidase (γ-GTP), and homocysteine were obtained from the health examination record. Blood samples were collected after subjects fasted for 12 hours. Biochemical measurements, including triglyceride, total cholesterol, HDL-cholesterol, LDL-cholesterol, AST, ALT, γ-GTP were assessed using an automated analyzer (TBA-200FR NEO; Toshiba, Tokyo, Japan) with enzymatic assays. Homocysteine levels had been measured using the IMx homocysteine assay (Axym 4011; Abbott, Dallas, TX, USA) following the procedure for the fluorescence polarization immunoassay.15) Hyperhomocysteinemia was set as a blood homocysteine concentration > 16 µmol/L, as suggested by Goldstein et al.16)
3. Statistics
Differences in general characteristics, BMI, and blood test results among three groups were compared as follows. The chisquare test with Bonferroni adjustment was used to examine categorical variables, including the presence of hypertension, diabetes, and smoking status. Continuous variables were analyzed using a one-way analysis of variance with posthoc Scheffe analysis. AST, ALT, triglyceride was used with a logarithmic transformation. In order to distinguish the difference between the flushing and nonflushing group, the quantity of alcohol consumed was grouped as <4; 4≤, <8; 8≤, <12; 12≤, <20; and ≥20 drinks/wk. These categories reflected real Korean habits, as one bottle of soju, popular among Koreans, contains four standard drinks. The frequencies of hyperhomocysteinemia for each drinking category in the facial flushing and nonfacial flushing groups were compared with those in the nondrinking group with the chi-square test. Additionally, a logistic regression analysis was conducted on the relationship between drinks consumed per week and hyperhomocysteinemia based on the nondrinking group by adjusting for the confounding factors of age, BMI, hypertension, smoking, log-transformed triglyceride, HDL-cholesterol, and γ-GTP. The analyses were conducted with SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
1. General Characteristics of the Study Subjects
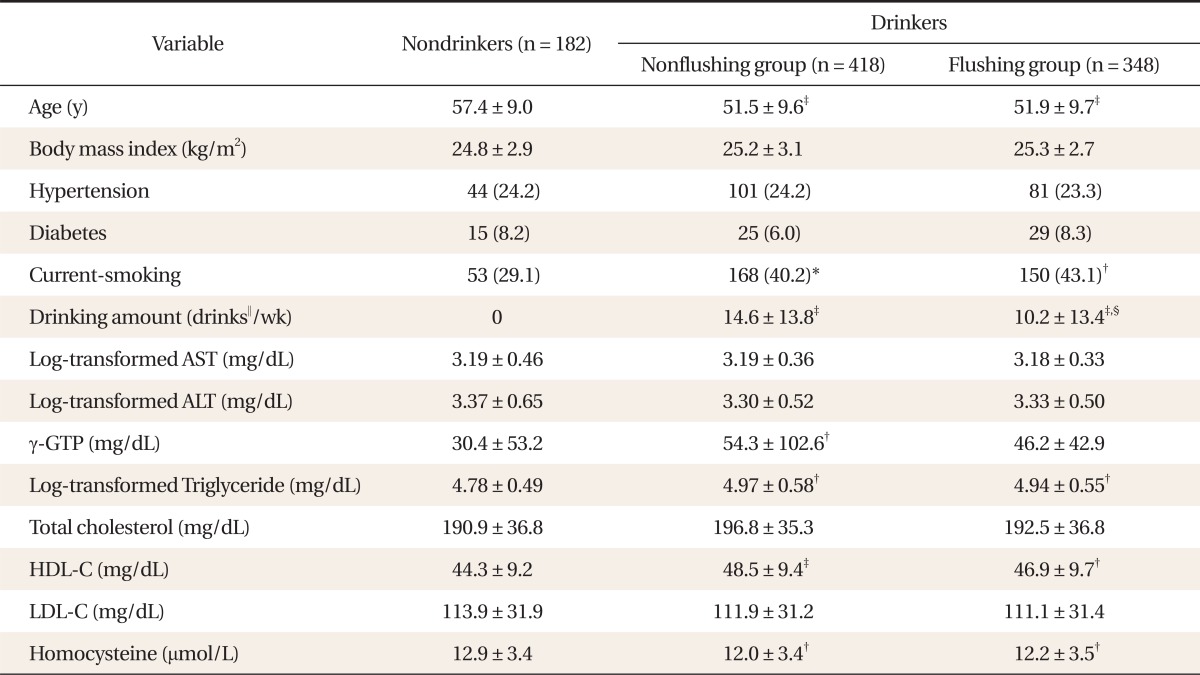
The mean ± SD age of the nondrinking, facial flushing, and nonfacial flushing groups was 57.4 ± 9.0, 51.9 ± 9.7, and 51.5 ± 9.6 years, respectively. The mean age was significantly lower in the facial flushing (P < 0.001) and nonfacial flushing (P < 0.001) groups than in the nondrinking group. In addition, the smoking rate was significantly higher in both the facial flushing (P < 0.01) and nonfacial flushing (P < 0.05) groups than in the nondrinking group. The nonfacial flushing and facial flushing groups did not differ significantly in BMI, hypertension frequency, and diabetes frequency compared with the nondrinking group. Alcohol consumption by the nonfacial flushing and facial-flushing groups was 14.6 ± 13.8 and 10.2 ± 13.4 drinks/wk, respectively.
Among the blood tests, log-transformed triglyceride levels were significantly higher in the nonfacial flushing (P < 0.01) and facial flushing (P < 0.01) groups than in the nondrinking group. Furthermore, HDL-choleserol levels were significantly higher in the nonfacial flushing (P < 0.001) and facial flushing (P < 0.01) groups than in the nondrinking group. γ-GTP levels were significantly higher in the nonfacial flushing (P < 0.01) groups than in the nondrinking group. Homocysteine concentrations of the nonfacial flushing (P < 0.01) and facial flushing (P < 0.01) groups were significantly lower than that of the nondrinking group. There was no significant difference between the nondrinking and drinking groups in the other blood tests, i.e., the log-transformed AST, log-transformed ALT, total cholesterol, and LDL levels (Table 1, Appendix 1). There was no significant difference between the nonfacial flushing group and facial flushing group in all variables except for the amount of alcohol consumed per week (P < 0.001).
2. Hyperhomocysteinemia Frequency Based on Drinks Consumed per Week
Hyperhomocysteinemia occurred in 16.5% (30/182) of the nondrinking group. In the nonflushing group, hyperhomocysteinemia occurred in 5.1% (5/99), 4.3% (3/70), 8.1% (3/37), 9.9% (7/71), and 11.3% (16/141) for the categories <4; 4≤, <8; 8≤, <12; 12≤, <20; and ≥20 drinks/wk, respectively. The frequency of hyperhomocysteinemia was significantly (P = 0.006 in <4 drinks; P = 0.01 in 4≤, <8 drinks) lower for those who drank <8 drinks/wk compared with the nondrinking group. For those who had ≥8 drinks/wk, the frequency of hyperhomocysteinemia did not differ significantly from the nondrinking group. Overall, as the amount of drinking increased, the frequency of hyperhomocysteinemia increased gradually.
For the facial flushing group, hyperhomocysteinemia occurred in 5.3% (8/150) of those who had <4 drinks/wk, which was significantly (P < 0.05) lower than in the nondrinking group. However, there was no significant difference in the frequency of hyperhomocysteinemia between the other drinking categories and the nondrinking group. Hyperhomocysteinemia occurred in 10.2% (6/59), 10.7% (3/28), 11.8% (4/34), and 10.4% (8/77) of those consuming 4 ≤, < 8; 8 ≤, < 12; 12 ≤, < 20; and ≥20 drinks/wk, respectively (Figure 1).
3. Risk of Hyperhomocysteinemia Based on Drinks Consumed per Week
From the results of the logistic regression analysis adjusted for confounding factors such as age, BMI, hypertension, diabetes, smoking, log-transformed triglyceride, HDL-cholesterol, and γ-GTP levels, based on the nondrinking group, the risk of hyperhomocysteinemia was significantly lower for those who had <4 (OR, 0.27; 95% CI, 0.10 to 0.74) and 4 ≤, <8 (OR, 0.21; 95% CI, 0.06 to 0.73) drinks/wk among the nonflushing group. However, there was no significant difference in risk of hyperhomocysteinemia between those who had ≥8 drinks/wk and the nondrinking group.
Adjusting for confounding factors, the risk of hyperhomocysteinemia was significantly lower (OR, 0.30; 95% CI, 0.13 to 0.68) in those who had <4 drinks/wk in the flushing group compared with the nondrinking group. However, there was no significant difference in risk of hyperhomocysteinemia between those who had ≥4 drinks/wk and the nondrinking group (Table 2).
DISCUSSION
This study investigated the relationship between weekly alcohol consumption and hyperhomocysteinemia based on facial flushing. To date, few studies have demonstrated that alcohol has a more negative effect on facial flushers than on nonfacial flushers in terms of hyperhomocysteinemia. Our study found that drinking might be associated with a lower risk of hyperhomocysteinemia among Koreans. Furthermore, this positive effect was demonstrated in the flushing group with lower alcohol consumption compared to the nonflushing group. That is why the adequate amount of drinking should be estimated lower in the flushing group than the nonflushing group.
Overall, the occurrence of hyperhomocysteinemia was lower in the drinking groups compared with the nondrinking group. Our results are consistent with those of previous studies demonstrating that drinking might lower the risk of hyperhomocysteinemia.17-19) The mechanism of hyperhomocysteinemia reduction is thought to be as follows: hyperhomocysteinemia might be reduced due to the functions of vitamin B6, vitamin B12, and folate20) found in beer and betaine21) found in wine, which facilitate homocysteine metabolism. However, the results of the current study contradict those of Ganji et al.22) in that there was no significant difference between the drinking groups and nondrinking group in subjects who had < 30 drinks/mo in the third National Health and Nutrition Examination Survey from 1988 to 1994. Furthermore, Ganji et al.22) showed that consuming >30 high-alcohol drinks elevated homocysteine levels. In interpreting this difference, the study subjects must be considered. Ganji et al.22) included men and women in their study, whereas our study included only men. Additionally, Ganji et al.22) targeted the general population, whereas our study examined people who had undergone a medical examination for health promotion at their own expense. Therefore, our subjects might be prone to healthier lifestyles.
Previous studies showed inconsistent results in the relationship between alcohol consumption with hyperhomocysteinemia. Our study was based on the facial flushing reaction which reflects individual vulnerability to alcohol. This study indicated that the risk of hyperhomocysteinemia was lower in the facial flushing group only for those who consumed <4 drinks/wk. This is half of the amount of those in the nonflushing group who exhibited significantly lower hyperhomocysteinemia rates. Therefore, the present study demonstrated that the occurrence of hyperhomocysteinemia differed based on facial flushing, despite the same alcohol consumption. The result of the current study implies the need for further studies to determine lower levels of alcohol consumption indicative of moderate drinking in the facial flushing group. Our study suggests that, in terms of the risk of hyperhomocysteinemia, <4 drinks/wk in the facial flushing group and <8 drinks/wk in the nonflushing group may be considered a moderate drinking amount for people of Korean ethnicity. Our results are consistent with the literature in that drinking has a greater negative effect on facial flushers than on nonfacial flushers who consume the same amount of alcohol.23-25) It is possible that in the facial flushing group, ALDH has low activity, so that acetaldehyde, a toxic substance, is not efficiently eliminated from the body and accumulates longer in facial flushers than nonfacial flushers. It is presumed that acetaldehyde is related to hyperhomocysteinemia, as it lowers the concentration of vitamin B6, which is involved in homocysteine metabolism.26) Additionally, acetaldehyde inhibits methionine synthase activation, which is necessary for transferring homocysteine into methionine.27)
One limitation of this study was that it was conducted by dividing the facial flushing group and nonfacial flushing group based on a questionnaire, although high sensitivity and specificity were reported for the questionnaire. Additionally, our study was a cross-sectional study instead of a prospective design. Furthermore, it targeted only males. Thus, larger studies including female subjects are required.
Despite these limitations, this study indicated that the risk of hyperhomocysteinemia is likely lowered by alcohol consumption based on drinking quantity, as lowering the risk of hyperhomocysteinemia differs depending on vulnerability associated with facial flushing. Therefore, facial flushing and the amount of alcohol consumed should be considered when counseling patients on drinking.
Notes
No potential conflict of interest relevant to this article was reported.
Appendices
Appendix 1
Characteristics of subjects in each drinking category
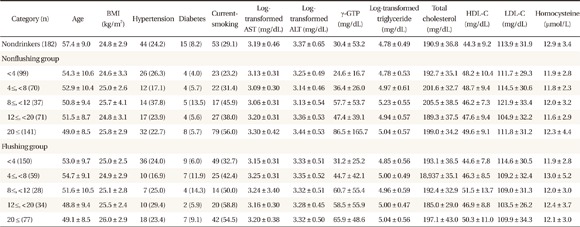
Values are presented as mean ± SD or number (%).
BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: gamma-glutamyl transferase, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol.