|
 |
- Search
| Korean J Fam Med > Volume 33(6); 2012 > Article |
In this section, we explain the statistical models and evaluation of heterogeneity among studies in meta-analysis, which appeared in the articles titled, "A comparison of efficacy and safety of nonsteroidal anti-inflammatory drugs versus acetaminophen in the treatment of episodic tension-type headache: a meta-analysis of randomized placebo-controlled trial studies" by Yoon et al.1) and "Use of proton pump inhibitor and risk of colorectal cancer: a meta-analysis of observational studies" by Ahn et al.2), both published in September 2012.
It is often the case that more than one investigation is performed to study a particular research question, often by different research groups. In some instances, results are seemingly contradictory, with some research groups reporting significant differences for a particular finding while other research groups report no significant differences.
The issue is, what is the appropriate way to combine evidence across all the studies so as to reduce sampling error and increase the power of the investigation, and in some instances to resolve the inconsistencies among the study results? The (statistical) method for accomplishing this is called meta-analysis.
It is controversial among research workers whether a fixed- or random-effects model should be used when performing a meta-analysis. Under a fixed-effects model, the between-study variance is ignored in computing the study weights and only the within-study variance is considered. In a random-effects model, both between- and within-study variation are considered. If the between-study variation is substantial relative to the within-study variation, then larger studies will be given proportionally more weight under a fixed-effects model than under a random-effects model. Hence, the pooled estimates under these two models may also be different.
It is argued that if there is substantial variation among the study results, then one should investigate the source of the heterogeneity (e.g., different study designs, etc.) and not report an overall pooled estimate. Others insist that between-study variation should always be considered in meta-analysis. A reasonable compromise might be to check for significant heterogeneity among study-specific estimates and use the decision rules as follows (Table 1).3)
Cochran's Q-test4) and Higgins' I2-statistic5) are frequently used to evaluate the statistical heterogeneity among study results. Cochran's Q-test provides us with a P-value which can be used directly to make a decision about the heterogeneity among studies. However, the small number of studies used in most meta-analyses gives us very low statistical power, thus the P-value of Cochran's Q-test usually shows no significance even though the heterogeneity among studies seems quite large. So we often use a significance level of 0.1 instead of a conventional 0.05 in meta-analysis. Higgins' I2-statistic is derived from Cochran's Q-statistic and defined as the proportion of variation due to the heterogeneity from total variation among studies.
I2 = ([Q - df] / Q) ├Ś 100 (%)
where df = k-1, k = number of studies.
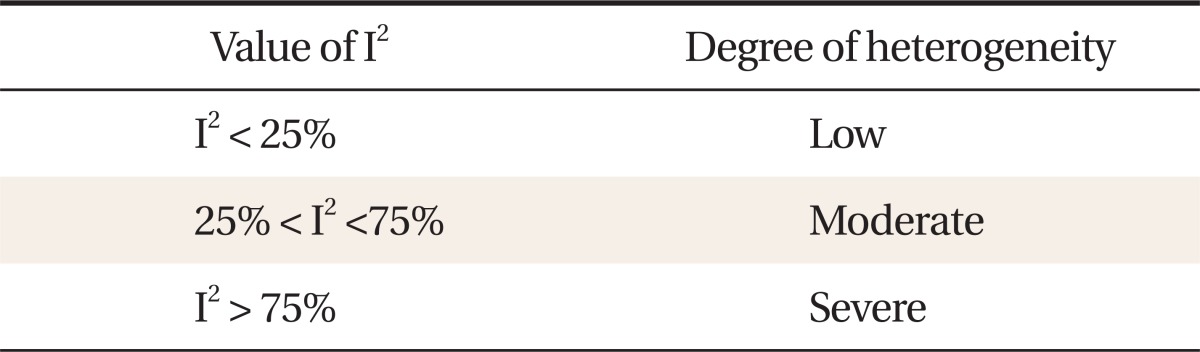
Values of I2 are between 0% and 100% as shown in table 2, and 0% represents no heterogeneity among studies (In this case, both fixed- and random-effects models have the same results). Negative values are treated as 0% and I2 = 50% is used as a criterion for choosing a statistical model.
References
1. Yoon YJ, Kim JH, Kim SY, Hwang IH, Kim MR. A comparison of efficacy and safety of non-steroidal anti-inflammatory drugs versus acetaminophen in the treatment of episodic tension-type headache: a meta-analysis of randomized placebo-controlled trial studies. Korean J Fam Med 2012;33:262-271. PMID: 23115700.



2. Ahn JS, Park SM, Eom CS, Kim S, Myung SK. Use of proton pump inhibitor and risk of colorectal cancer: a meta-analysis of observational studies. Korean J Fam Med 2012;33:272-279. PMID: 23115701.



3. Rosner B. Fundamentals of biostatistics. 2000. 5th ed. Pacific Grove: Duxbury.
5. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 2002;7:51-61. PMID: 11822262.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,374 View
- 15 Download
- Related articles in KJFM
-
Comments on Statistical Issues in January 20162016 January;37(1)
Comments on Statistical Issues in November 20152015 November;36(6)
Comments on Statistical Issues in January 20152015 January;36(1)
Comments on Statistical Issues in September 20152015 September;36(5)