The Relationship between Smoking Level and Metabolic Syndrome in Male Health Check-up Examinees over 40 Years of Age
Article information
Abstract
Background
The objective of this study was to investigate the relationship between smoking and metabolic syndrome in men.
Methods
This cross-sectional study included 1,852 men over age 40 who underwent health screening from April 2009 to December 2010. We classified them into three smoking levels as non-, intermediate-, and heavy-smoker, considering their smoking status (non, ex, current) and amount (0, 1-29, ≥30 pack year [PYR]). The relationship between smoking level and metabolic syndrome was analyzed by logistic regression analysis, after covariates (age, body mass index, education, house income, alcohol intake, and physical activity) were controlled.
Results
The proportions of non-, intermediate-, and heavy-smokers were 31.8%, 56.2%, and 12.0%, respectively. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for metabolic syndrome were 1.0, 1.58 (1.09-2.23), 1.92 (1.29-2.81) in non-, intermediate-, and heavy-smokers, respectively. For heavy-smokers compared with non-smokers, ORs and 95% CIs of a lower high density lipoprotein cholesterol, higher triglyceride, and higher fasting glucose were 2.47 (1.63-3.74), 1.71 (1.17-2.52), and 1.43 (1.02-2.00), respectively. In current-smokers, we divided into three subgroups according to PYR, and compared with 1-19 PYR, ORs and 95% CIs of 20-29 PYR and ≥30 PYR for metabolic syndrome were 2.07 (1.14-3.74) and 3.06 (1.66-5.62), respectively.
Conclusion
This study showed a positive dose-response relationship between smoking level and metabolic syndrome in men.
INTRODUCTION
For prevention and management of chronic diseases, improvement of health behaviors such as smoking, drinking, diet, and physical activity are most effective.1) Smoking, known as the worst health behavior, is not only a risk factor that causes cardiovascular disease and malignant tumor,2,3) but is also related to metabolic disease such as diabetes and obesity,4) and even passive smoking is damaging to health.5) It has been demonstrated that smoking is associated with reduced insulin sensitivity and diabetes risk,6) impaired lipid metabolism,7) abdominal obesity by abdominal fat accumulation,8) increased blood pressure,9) oxidative stress, and artherosclerosis.10)
Metabolic syndrome (MS), a surrogate indicator of type 2 diabetes and cardiovascular disease, refers to a group of disease conditions including abdominal obesity, dyslipidemia, high blood pressure, and insulin resistance.11) As mentioned above, most metabolic effects of smoking are overlapped with (those of) MS. For these reasons, it has been increasing interest that the relationship between smoking and cardiovascular was mediated by MS.12)
Although a few studies suggested that smoking may have a preventive effect on MS,13,14) but recently it turns out to be a false result caused by the confounder effect of obesity, the preventive effect of smoking was disappeared after weight was controlled.15) As a result, smoking is an independent risk factor for MS and showed a dose-response relationship with MS.16) Long-term smoking causes insulin resistance and increase the risk of MS and diabetes, regardless of current or past smoking.5,17)
Smoking level has two components: one is 'smoking status,' referring to non-smoking, ex-smoking, and current-smoking and the other is 'smoking amount,' referring to non-smoking, intermediate-smoking, and heavy-smoking according to pack year (PYR: "how many years did you smoke about 1 pack per day?"; the concept of whole-life smoking amount). However, there are limitations in this oversimplified classification; smoking status is likely to underestimate past-smoking or overestimate current-smoking, and smoking amount also ignores time effect on human body because low-grade inflammation, such as MS, is able to recover by effective intervention.
Therefore, in this cross-sectional study, we divided study participants into three groups according to smoking level as non-(never smoking), heavy-(current smoking and ≥30 PYR), and intermediate-smoker (other), considering smoking status and smoking amount, then we investigated the relationship between smoking and MS.
METHODS
1. Study Population
One thousand nine hundred and sixty men over age 40 who underwent health examination from April 2009 to December 2010 agreed to sign the informed-consent for this study. One hundred and eight persons were excluded if they had the target disease of MS (myocardial infarction, angina, stroke, and diabetes; n = 74) or incomplete answers (n = 34). As a result, a total of 1,852 persons were included.
2. Methods
Demographic and lifestyle data were collected by a trained interviewer using a questionnaire, and anthropometric and blood test were conducted concurrently.
1) Smoking level classification
Reliable and persuasive estimates are required to assess smoking level. Two aspects of smoking were considered, 'smoking status' (non-, ex-, current smoking) and 'smoking amount' (PYR: average daily smoking amount [pack] × smoking period [year]; 0 PYR, 1-29 PYR, and ≥30 PYR). We classified study participants into three groups as non-(never smoking), heavy-(current smoking and ≥30 PYR), and intermediate-smokers (other) according to smoking level.
2) Definition of metabolic syndrome
For the definition of MS, the criteria of the modified National Cholesterol Education Program Adult Treatment Panel III (2007)18) were followed when more than three of the five conditions were met: (1) waist circumference ≥90 cm; (2) blood pressure ≥130/85 mm Hg or medication; (3) triglyceride ≥150 mg/dL or medication; (4) fasting glucose ≥100 mg/dL or medication; (5) high density lipoprotein (HDL) cholesterol <40 mg/dL.
3) Socioeconomic status
Education was divided into nine levels: no education, withdrawn from elementary school, elementary graduate or withdrawn from middle school, middle school graduated or withdrawn from high school, high school graduate, college graduate, withdrawn from university, university graduate, and graduate school. House income (monthly) was divided into eight levels: <0.5, 0.5-1.0, 1.0-1.5, 1.5-2.0, 2.0-3.0, 3.0-4.0, 4.0-6.0, and ≥6.0 million won.
4) Lifestyle factors
Smoking, drinking, and leisure time physical activities of study participants were examined. As for drinking, kinds of alcohol were listed including soju, beer, makgulli, sake, wine, whisky, champaign, and others. And average daily alcohol consumption for the last year was calculated by monthly drinking frequency and average amount of one drink. Using the Korean version of the Minnesoita Leisura-Time Physical Activity Questionnaire (MLTPAQ),19) energy expenditure (kcal/d) was calculated as the metabolic equivalent of task that is unique for each physical activity during leisure time.
5) Anthropometric measurement and blood test
Height and weight were measured in a standing position to obtain body mass index (BMI, kg/m2). Waist circumference was measured at the level of the iliac crest in a standing position. Systolic blood pressure and diastolic blood pressure were measured after relaxing for more than 5 minutes, and the average values were obtained after repeated measurements. As for blood testing, the blood samples were collected from the brachial vein after fasting for at least 8 hours, and fasting blood glucose, triglyceride, total cholesterol, HDL cholesterol, high-sensitivity C-reactive protein (hs-CRP), and gamma-glutamyltransferase (GGT) were measured.
3. Statistical Analysis
IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) were used for data analysis and a P-value <0.05 was regarded as significant. Logistic regression was performed to obtain odds ratios (ORs) and 95% confidence intervals (95% CIs) of smoking with MS, after adjusting for age, BMI, education, house income, alcohol amounts (g/d), and physical activity (kcal/d) as covariates. Chi-square tests and analysis of variance were conducted to compare education, house income, drinking, physical activity, BMI, blood test results, and MS with smoking level. The participants who already had hypertension medication (285 persons) were considered to have hypertension components of MS regardless of measured value. We excluded 285 persons after the relationship of smoking and high blood pressure was analyzed.
RESULTS
1. General Characteristics of Study Participants
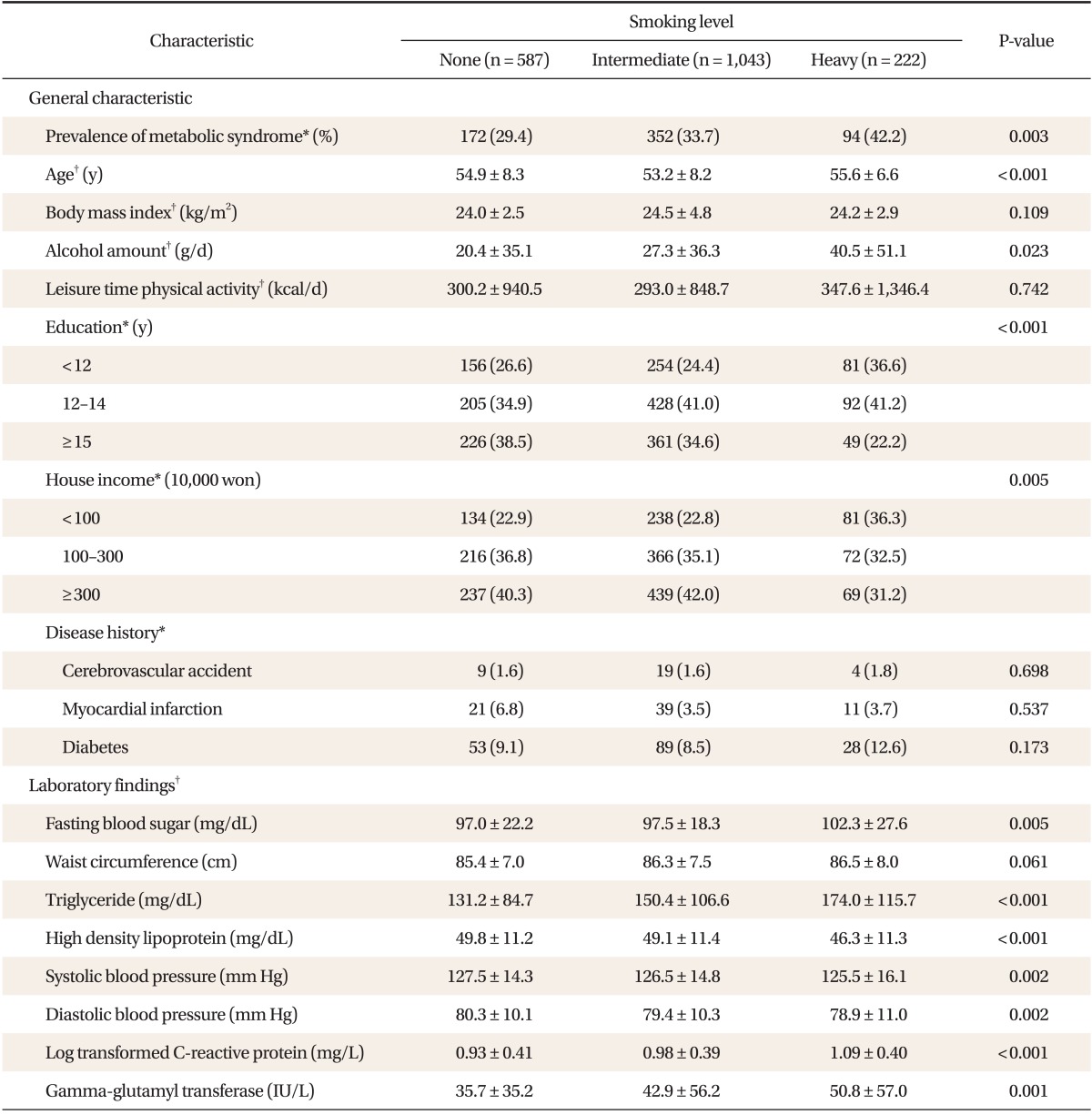
Of the 1,852 subjects, the proportions of non-, intermediate-, and heavy-smoker were 31.8% (587 persons), 56.2% (1,043 persons), and 12.0% (222 persons), respectively and the prevalence of MS were 29.4%, 33.7%, and 42.2%, respectively. The prevalence of MS was increased as smoking level increased. Education and house income level was inversely associated with smoking level, and daily alcohol consumption increased with smoking level, but physical activity was not significant. Fasting glucose, triglyceride, and HDL were worse, but blood pressure improved as smoking increased, hs-CRP (log transformed) and GGT, which reflect low-level inflammation, increased as smoking increased (Table 1).
2. Association of Smoking with Components of Metabolic Syndrome
Smoking showed a positive relationship with components of MS. After adjusting for covariates (age, BMI, alcohol amounts, daily physical activity, education, house income), OR was set as 1 in non-smoker, and odds ratios (ORs) and 95% confidence intervals (95% CIs) of intermediate- and heavy-smokers for fasting glucose were 1.11 (0.81-1.51), 1.43 (1.02-2.00), for triglyceride 1.47 (1.03-2.11), 1.71 (1.17-2.52), and for HDL 2.07 (1.41-3.02), 2.47 (1.63-3.74) (Table 2).
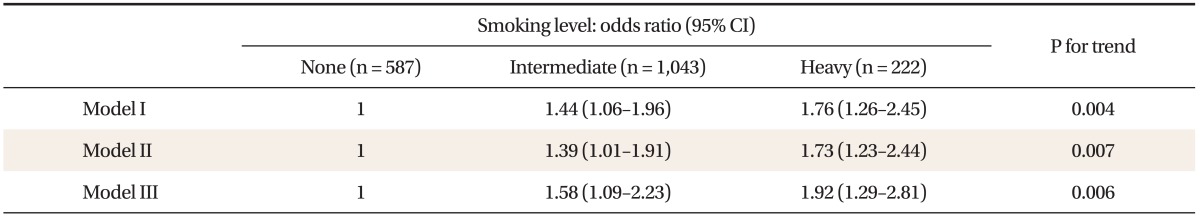
3. Association of Smoking with Metabolic Syndrome
OR was set as 1 in non-smokers without adjusting for covariates, and ORs and 95% CIs for MS in intermediate- and heavy-smokers were 1.44 (1.06-1.96) and 1.76 (1.26-2.45) (model I). After age and BMI was adjusted, ORs and 95% CIs in intermediate- and heavy-smokers were 1.39 (1.01-1.91) and 1.73 (1.23-2.44) (model II). After age, BMI, education, house income, alcohol amounts (g/d), and physical activity (kcal/d) were adjusted for, ORs and 95% CIs in intermediate- and heavy-smokers were 1.58 (1.09-2.23) and 1.92 (1.29-2.81) (model III). Thus, the prevalence of MS linearly increased as smoking level increased (Table 3).
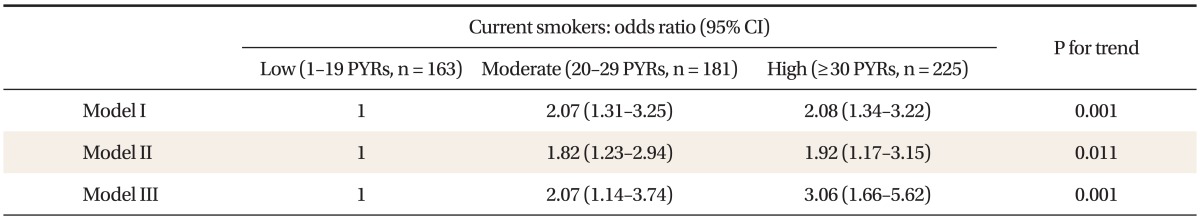
4. Association of Smoking Amount (Pack Year) with Metabolic Syndrome in Current Smokers
Among participants, proportions of non-, ex-, and current-smoking were 29.3% (542 persons), 40.0% (741 persons), and 30.7% (569 persons), respectively. After non-, and ex-smokers were excluded, we divided current-smokers into three subgroups according to PYR (1-19 PYR; 20-29 PYR; ≥30 PYR), and compared the prevalence of MS. When OR was set as 1 in the 1-19 PYR, after adjusting for covariates (age, BMI, alcohol amounts, physical activity, education, and house income), ORs and 95% CIs in 20-29 PYR and ≥30 PYR were 2.07 (1.14-3.74) and 3.06 (1.66-5.62), respectively (Table 4).
DISCUSSION
In this study, the risk of MS showed a dose-response relationship ORs 1.58 and 1.92 in intermediate- and heavy-smokers compared with non-smokers. These results were consistent with other domestic and foreign studies.14,20) In Korea National Health and Nutrition Examination Survey (KNHANES, 1998), ORs and 95% CIs were 1.2 (0.9-1.6), 1.5 (1.2-2.1), and 1.6 (1.1-2.1) in ex-, intermediate-, and heavy-smokers, respectively, compared with that of non-smokers.16) In another three-year follow-up domestic study, relative risk and 95% Cis were 1.90 (1.21-3.00) in current smoker (≥1 pack/d) and 1.66 (1.18-2.33) in long term smoker, compared with non-smoker.15) In a Japanese study conducted on white-collar workers, ORs and 95% CIs were 1.30 (1.00-1.68), 1.17 (0.88-1.56), and 1.66 (1.24-2.20) in ex-, intermediate-, and heavy-smokers, compared with non-smokers.18) This cross-sectional study showed that hs-CRP20) and GGT,21) which are low-level inflammation markers, also linearly increased with smoking level.
Biologically, the most important factor in the relationship between smoking and MS is insulin resistance caused by complicated reactions of cotinine, carbon mono oxide (CO), cortisol, the sympathetic nervous system, and growth hormone. Cotinine, a metabolite of nicotine, induces a low-level inflammation response,22) and nicotine, CO, cortisol, the sympathetic nervous system, and growth hormone activate anti-estrogen effects and finally reduce insulin sensitivity.19,23)
In addition, socioeconomic factors such as education and income, and lifestyle factors such as drinking and physical activity were closely correlated with the incidence of metabolic syndrome.24,25) Their results were consistent with ours.
Among components of MS, HDL, triglyceride, and glucose were associated with smoking level, and our results were consistent with those of other studies showing that smoking decreases HDL, increases LDL, and increases insulin resistance.7,26) In previous experimental studies and prospective studies, smoking increased arterial hardness and blood pressure,10,27,28) and promoted visceral fat accumulation,29) thereby worsening abdominal obesity related diseases.30,31)
However, There was no significant relationship among smoking, blood pressure, and abdominal obesity in this study. We considered limitations of a cross-sectional study that could explain the difference. In addition, there were controversies over the relationship between smoking and blood pressure.20,27,31)
The limitations of this study are as follows. First, this study was conducted in a health promotion center of single university hospital. Thus, generalization of the results is impossible. However, compared to KNHANES (1998), the participants of this study were older, more educated, and smoked less (in KNHNES, ≥20 years, proportion of over high school graduate was 27.2% and proportion of heavy smoker was 16.3%; in this study, ≥40 years, over high school was 38.5% and heavy smoker was 12.0%). We could infer null effects between age and education for smoking level that attenuated selection bias. Second, as a cross-sectional study, it could not explain the causality between smoking and MS. Third, information and recall bias caused by willful and illusory memory could not be excluded.
Despite the limitations, one strength of this study is that the smoking assessment had multiple components, including both smoking status and amount, and the relationship between smoking and MS was analyzed after controlling various variables.
Notes
No potential conflict of interest relevant to this article was reported.