Efficacy of Vitamin C Supplements in Prevention of Cancer: A Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
Background
Previous randomized controlled trials (RCTs) have reported inconsistent findings regarding the association between vitamin C supplementation and the risk of cancer.
Methods
We performed a meta-analysis of RCTs to investigate the efficacy of vitamin C supplements for prevention of cancer. We searched the PubMed, EMBASE, and Cochrane Library databases in November 2014 using common keywords related to vitamin C supplements and cancer.
Results
Among 785 articles, a total of seven trials were identified, which included 62,619 participants; 31,326 and 31,293 were randomized to vitamin C supplementation and control or placebo groups, respectively, which were included in the final analysis. A fixed-effects meta-analysis of all seven RCTs revealed no significant association between vitamin C supplementation and cancer (relative risk, 1.00; 95% confidence intervals, 0.95-1.05). Similarly, subgroup meta-analysis by dose of vitamin C administered singly or in combination with other supplements, follow-up period, methodological quality, cancer mortality, gender, smoking status, country, and type of cancer also showed no efficacy of vitamin C supplementation for cancer prevention.
Conclusion
This meta-analysis shows that there is no evidence to support the use of vitamin C supplements for prevention of cancer.
INTRODUCTION
Previous animal and in vitro studies have indicated that free radicals such as reactive oxygen species (ROS) can cause cellular damage and lead to cancer by altering cellular regulatory pathways.12) Vitamin C has been considered an antioxidant that can prevent ROS-induced cellular damage.34) However, the efficacy of vitamin C supplements for prevention of cancer has been controversial for decades.
In 1976, a possible therapeutic effect of high-dose vitamin C supplementation in 100 patients with advanced cancer was reported in a non-randomized clinical trial by Cameron and Pauling,56) compared to a control group of 1,000 untreated and matched patients. However, two subsequent randomized placebo-controlled trials (RCTs) by Creagan et al.7) and Moertel et al.8) in 1979 and 1985 reported no beneficial effect of high-dose vitamin C therapy in patients with advanced cancer.
Since then, several RCTs have shown inconsistent findings regarding the effects of vitamin C supplementation on the risk of cancer. In 2006, a systematic review of four RCTs indicated that vitamin C supplements did not increase survival in cancer patients.9) Similarly, a meta-analysis of several RCTs published in 2008 reported that vitamin C supplementation given singly or in combination with other antioxidant supplements had no effect on the prevention of gastrointestinal cancers.10) Additionally, a meta-analysis by Jiang et al.11) indicated that vitamin C supplementation had no significant effect on prevention of prostate cancer. Although additional large RCTs have been published, no comprehensive meta-analysis on this issue has been reported so far.
The aim of this study was to perform a meta-analysis of RCTs in order to assess the efficacy of vitamin C supplements for cancer prevention. Subgroup meta-analyses were also performed by vitamin C supplement dose, follow-up period, methodological quality, outcome, funding source, gender, smoking status, country, and type of cancer.
METHODS
1. Data Search
We searched the PubMed, EMBASE, and Cochrane Library databases in November 2014, using keywords related to the efficacy of vitamin C supplements for prevention of cancer. The keywords were 'vitamin C' or 'ascorbic acid,' 'cancer,' and 'randomized controlled trials.' The bibliographies of relevant articles were also searched. We included only RCTs that both reported the efficacy of vitamin C supplementation on cancer prevention and compared an intervention group with a control group. The main outcome measures included cancer incidence and mortality. We did not restrict the language of publication.
2. Study Selection and Data Acquisition
Two authors (BL and SKM) independently assessed the eligibility of all trials identified from the databases and bibliographies. Disagreements on study eligibility were resolved by consensus or through consultation with the other author (SWO). From the studies selected for the final analysis, we retrieved the study name (with first author and year of publication), country, study design, duration of supplementation (in years), pharmaceutical industry funding, participants (number and underlying conditions), contents of intervention and control, type of cancer, and the number of outcomes and participants in each group.
3. Assessment of Methodological Quality
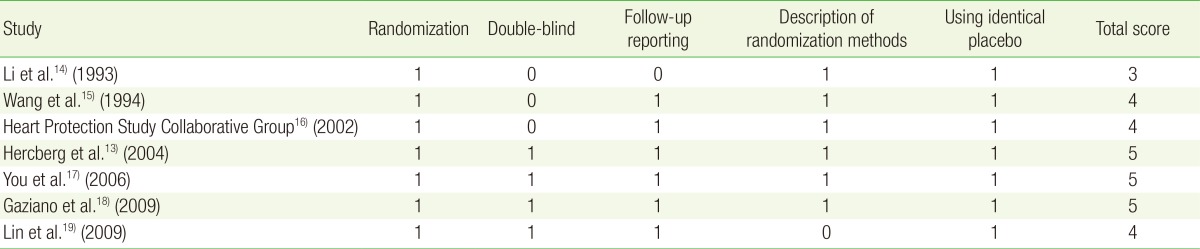
To evaluate the methodological quality of the trials, we used the Jadad scale, a validated scale for RCTs developed by Jadad et al.12) With a total of five points, this scale assigns a point each for randomization (1 point if randomization is described; additional 1 point if randomization process such as table of random numbers or computer-generated randomization is described), double-blind (1 point if described as double-blind; additional 1 point if masking such as identical placebo was used), and follow-up (1 point for reporting the numbers and reasons for withdrawal in each group) in the final report of a randomized controlled trial.12) In assessing the quality, RCTs with scores of 4 or less were considered low quality, while scores of 5 were high quality.
4. Main and Subgroup Analysis
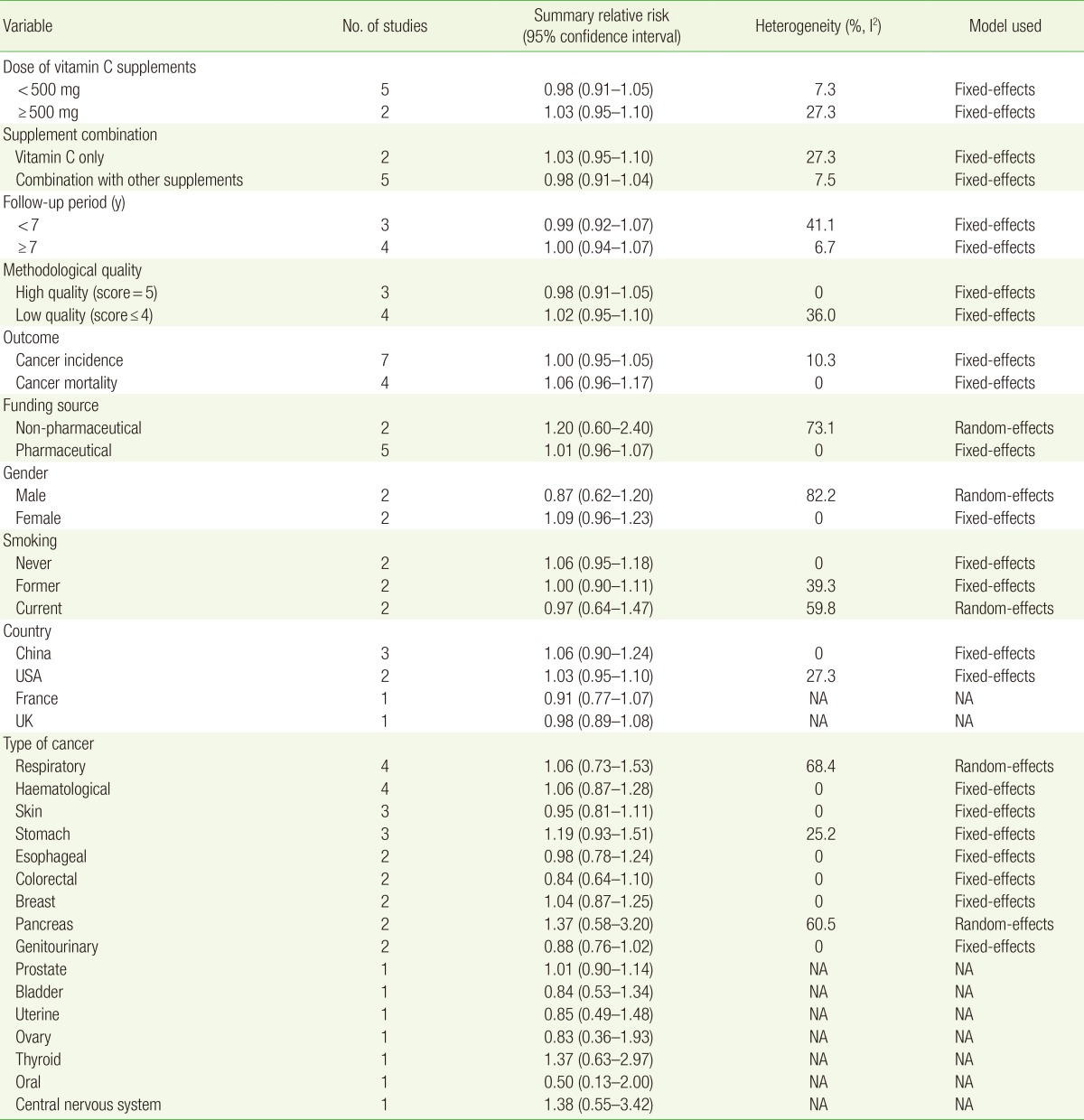
The main analysis examined the efficacy of vitamin C supplements on cancer incidence or mortality. We also performed subgroup meta-analyses by dose of vitamin C (<500 mg vs. ≥500 mg), supplement combination (vitamin C only vs. combination with other supplements), follow-up period (<7 years vs. ≥7 years), methodological quality (high quality vs. low quality), outcome (cancer incidence vs. cancer mortality), gender (male vs. female), funding source (non-pharmaceutical vs. pharmaceutical), smoking status (never vs. former vs. current), participant nationality (China vs. USA vs. France vs. UK), and type of cancer.
5. Statistical Analyses
Trial heterogeneity of were assessed using Higgins I2, which measures the total variation across trials for a specific percentage. I2 was calculated as follows:
Either fixed- or random-effects models served as the basis for calculation of pooled relative risk (RR) with 95% confidence intervals (CIs). Without substantial heterogeneity, the pooled RR with 95% CIs were reported based on the fixed-effects models; in this case the summary estimates are similar for both the fixed- and random-effects models. With substantial heterogeneity, the summary estimates from the random-effects model were reported because often CIs are larger in the random-effects model than in the fixed-effects model. The Mantel-Haenszel and the DerSimonian and Laird methods were used for fixed- and random-effects models, respectively. We used Stata SE ver. 12.0 (Stata Co., College Station, TX, USA) for statistical analysis.
RESULTS
1. Trial Selection
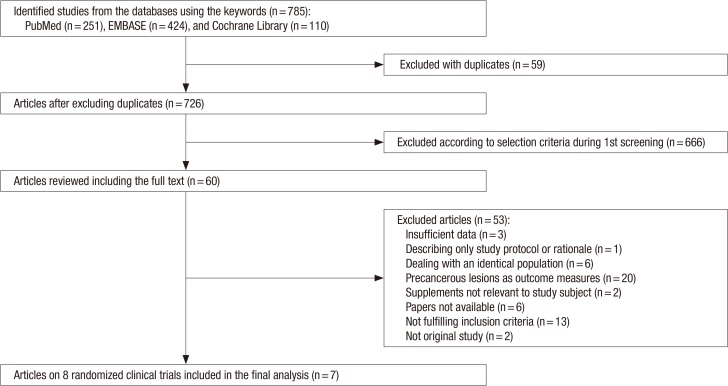
As shown in Figure 1, a total of 785 articles were identified after searching three databases (PubMed, EMBASE, and the Cochrane Library). After excluding 59 duplicated articles and 666 articles that did not satisfy the selection criteria, the full texts of 60 articles were reviewed by two authors of this study. Among those, 53 articles were excluded for the following reasons: insufficient data (n=3), describing only the study protocol or rationale (n=1), identical population (n=6), precancerous lesions as outcome measures (n=20), supplements not relevant to study subject (n=2), no available papers (n=6), not fulfilling inclusion criteria (n=13), or not an original study (n=1). A total of seven trials were included in the final analysis.
2. General Characteristics of Included Trials
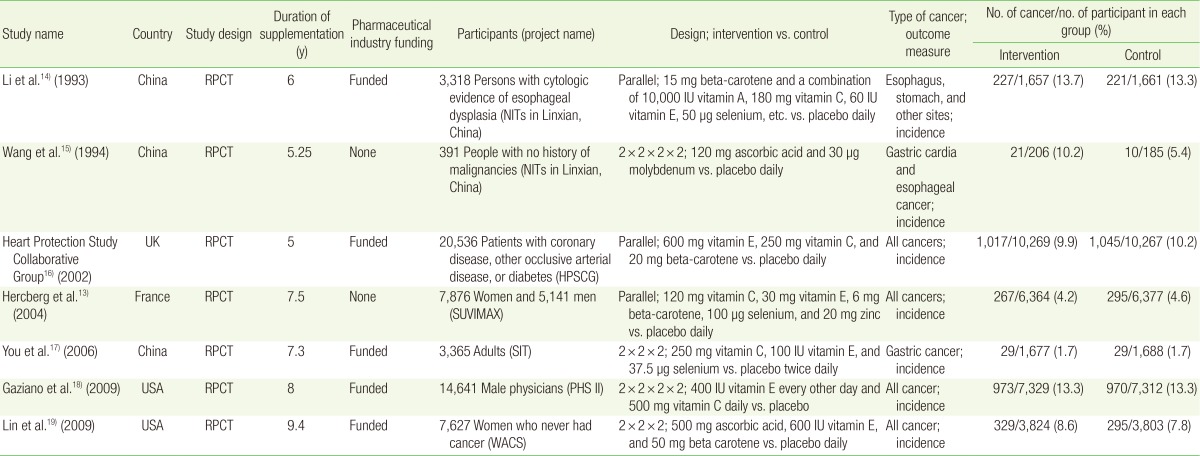
The seven trials included 62,619 participants with 31,326 and 31,293 randomized to the vitamin C supplementation and control groups, respectively. Among trials that reported age, the participants ranged from 35 to 80 years. One RCT that used low-dose vitamin C supplements in combination with other supplements showed a reduction in cancer incidence and mortality.13) The remaining six RCTs showed no significant effect of vitamin C supplementation.141516171819)
Table 1 shows the general characteristics of the seven RCTs included in the final analysis. The included trials were published from 1993 through 2009. The studies were conducted in China (n=3), the US (n=2), France (n=1), and the UK (n=1). Both the treatment and the follow-up periods ranged from 5 to 9.4 years. All trials used placebos in the control group. Five trials received funding from the pharmaceutical industry; the remaining two did not. The number of participants in each trial ranged from 391 to 20,536. Among the seven trials, two used only vitamin C supplements as intervention; the rest used vitamin C supplements in combination with other vitamin or antioxidant supplements. The daily vitamin C doses, either singly or in combination with other supplements, varied from 120 to 500 mg.
3. Methodological Quality
Table 2 shows the quality of study methodology included in the final meta-analysis. The quality scores ranged from 3 to 5. Among the seven trials, three trials received a score of 5, three trials received a score of 4, and one trial received a score of 3.
4. Overall Efficacy in All Seven Trials
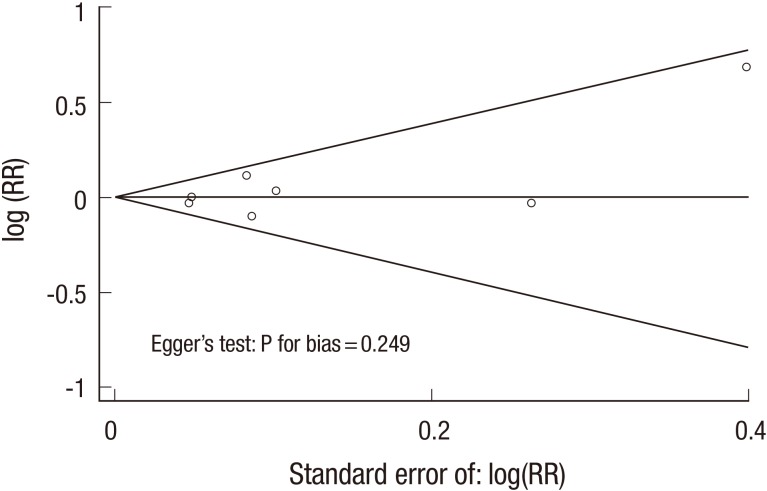
In a fixed-effects meta-analysis of all trials, vitamin C supplementation had no significant efficacy on cancer incidence, when compared with the control group (RR, 1.00; 95% CIs, 0.95-1.05; I2=10.3%) (Figure 2). The Begg's funnel plot was almost symmetrical, and the Egger's test showed no bias (P for bias=0.249) (Figure 3).
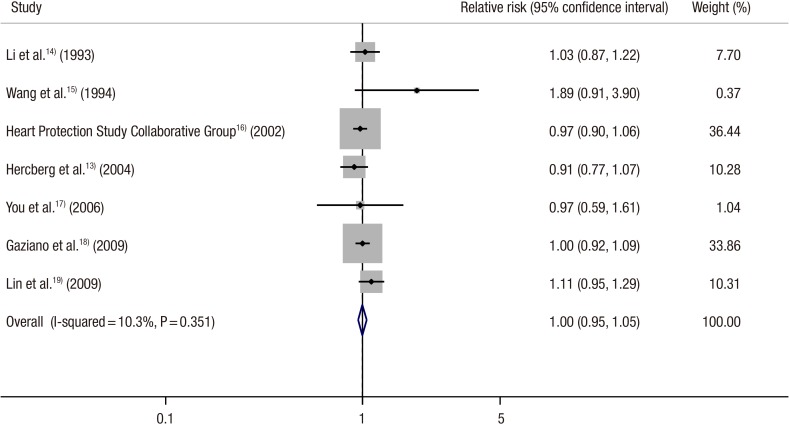
Effect of vitamin C supplement and control on cancer incidence and mortality in randomized controlled trials (n = 7).
5. Subgroup Meta-Analyses
Table 3 shows subgroup meta-analyses of the efficacy of vitamin C supplements on cancer according to various factors. There was no significant association between vitamin C supplementation and cancer in subgroup meta-analyses by vitamin C dose administered singly or in combination with other supplements, follow-up period, methodological quality, cancer mortality, gender, smoking status, country, and type of cancer.
DISCUSSION
In this meta-analysis of seven RCTs, we found no significant association between vitamin C supplementation and cancer risk. In addition, no efficacy of vitamin C supplements was found for the prevention of cancer in subgroup analyses according to vitamin C dose administered singly or in combination with other supplements, follow-up period, methodological quality, cancer mortality, gender, smoking status, country, and type of cancer.
Vitamin C is an electron donor that non-enzymatically stabilizes ROS by reducing them; in this process, vitamin C is oxidized to an ascorbyl radical. Unlike other dangerous free radicals, the ascorbyl radical is short-lived, with a half-life of 10-5 seconds, and is relatively unreactive.20) Shortly after donating an electron, the ascorbyl radical donates another electron and is further oxidized to dehydroascorbic acid. These two oxidized forms of vitamin C can be reduced back to the original state by at least three different enzyme pathways and reducing biological compounds.21) However, not all oxidized ascorbic acid is recovered. Some dehydroascorbic acid is metabolized by hydrolysis and is lost to other biological compounds.22) Therefore, the stability, reducibility, and reusability of oxidized ascorbic acid explain why vitamin C is a preferred antioxidant.
The findings of our analysis were consistent with those of previous meta-analyses of RCTs, which concluded no association between vitamin C and prostate, colorectal, and gastrointestinal cancers.91023) Our findings, however, were inconsistent with those of previously published preclinical in vivo or in vitro studies on this issue.242526) In 2005, an in vitro experiment conducted by Abdel-Latif et al.27) suggested the efficacy of vitamin C supplements in facilitating apoptosis of esophageal cancer cells when used in conjunction with chemotherapy. Also, in 2010, Pollard et al.24) showed that pharmacological doses of ascorbic acid could greatly reduce both tumor growth and cancer metastases in the prostate and lungs of rats.
This discrepancy in findings between preclinical in vivo or in vitro studies and RCTs might be due to the fact that preclinical studies may not represent biological responses in the human body.28) Anti-carcinogenic antioxidants in animals could be carcinogenic to humans. For instance, experimental studies indicated that beta-carotene reduces the risk of cancer incidence,29) but may act as a pro-oxidant under chronic oxidative stress such as tobacco exposure in humans and lead to lung cancer.3031)
In 2009, Bandera et al.32) reported a meta-analysis of 12 case-control studies, indicating a strong inverse association between antioxidant vitamins and the risk of endometrial cancer. Another meta-analysis of 13 cohort studies conducted by Park et al.33) showed a slight inverse association between vitamin C supplementation and the risk of colon cancer.
A similar discrepancy in findings between observational epidemiologic studies and RCTs might be explained by recall and selection biases common in retrospective studies such as case-control studies. As for recall bias, recollection of diet may differ between cancer patients and healthy controls; cancer patients are prone to report an unhealthy diet, while healthy controls report otherwise.3435)
In evaluating the association between use of antioxidant supplements and risk of cancer, cohort studies are less biased than case-control studies, but causation cannot be sufficiently supported.3637) The diet assessment tools used in cohort studies might not precisely evaluate participants' long-term vitamin C consumption. In addition, use of vitamin C supplements in trials differs from the intake of fruit and vegetables rich in vitamin C in cohort studies, which contain other vitamins and micronutrients in addition to vitamin C. That is, vitamin C might have beneficial effects when administered in combination with other nutrients.
It is noteworthy that our findings are similar to those of the previously published meta-analysis of RCTs on the association between antioxidant supplementation and cancer and mortality. In 2007, Bjelakovic et al.38) reported the results of meta-analysis of 47 high-quality trials involving 180,938 participants. Their findings showed that vitamin A, vitamin E, or beta-carotene supplementation increased mortality while vitamin C and selenium were not significantly associated with mortality.38) The authors proposed that removing free radicals through antioxidant supplementation might impede some major defensive mechanisms such as apoptosis, phagocytosis, and detoxification, thus increasing mortality. Similarly, in 2010, Myung et al.39) reported that antioxidant supplements showed no significant effect on primary and secondary cancer prevention. Furthermore, in subgroup meta-analysis of four RCTs by type of cancer revealed increased risk of bladder cancer.39)
Regarding the efficacy of high-dose vitamin C therapy, the different findings between Cameron and Pauling56) and Creagan et al.7) and Moertel et al.8) have marked an ongoing controversy. There are two important differences between the two trials. First, Cameron and Pauling56) used both intravenous and oral administrations of vitamin C, while Creagan et al.7) and Moertel et al.8) only used oral administration. In general, the concentration of ascorbic acid was much higher when administered intravenously than orally. Furthermore, higher concentrations of vitamin C have been found to accumulate in tumors compared to normal tissues.404142) Likewise, laboratory data suggested the toxicity of high-dose vitamin C in cancer cell lines.434445) Chen et al.46) suggested that elevated concentrations of ascorbic acid might increase formation of hydrogen peroxide (H2O2), which may be toxic to tumor cells. This suggests a possible explanation for why Cameron and Pauling56) found high-dose vitamin C supplementation to be therapeutic, while Moertel et al.8) did not. The other difference is that the studies by Cameron and Pauling56) were not RCTs, while those by Creagan and Moertel were RCTs. Cameron and Pauling56) used matched controls without randomization. Additional large randomized, double-blind, placebo-controlled trials are necessary to investigate the effects of oral or intravenous administration of high-dose vitamin C therapy on cancer risk.
Our study has several limitations. First, we were unable to investigate the beneficial effects of vitamin C supplements on cancer in patients with vitamin C deficiency because few RCTs included these populations. Further trials are needed to explore this association. Second, our findings should not be directly translated to the effects of fruit and vegetables rich in vitamin C. Last, the participants included in our meta-analysis were mostly 50-60 years of age. Thus, our findings cannot be generalized to younger populations. Further RCTs in younger participants are therefore necessary.
In summary, our meta-analyses of RCTs showed no efficacy of vitamin C supplementation for prevention of cancer. Further large-scale, randomized controlled trials are necessary to confirm our findings.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.