Relationship between Chronic Kidney Disease and Depression in Elderly Koreans Using the 2013 Korea National Health and Nutrition Examination Survey Data
Article information
Abstract
Background
Depression is prevalent in patients with chronic kidney disease (CKD) and continues to increase in elderly adults. Therefore, the aim of our study was to examine the relationship between CKD and depression in older patients.
Methods
We conducted a cross-sectional study based on 2013 Korea National Health and Nutrition Examination Survey data. In total, data of 973 subjects aged ≥65 years were analyzed, and the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Results
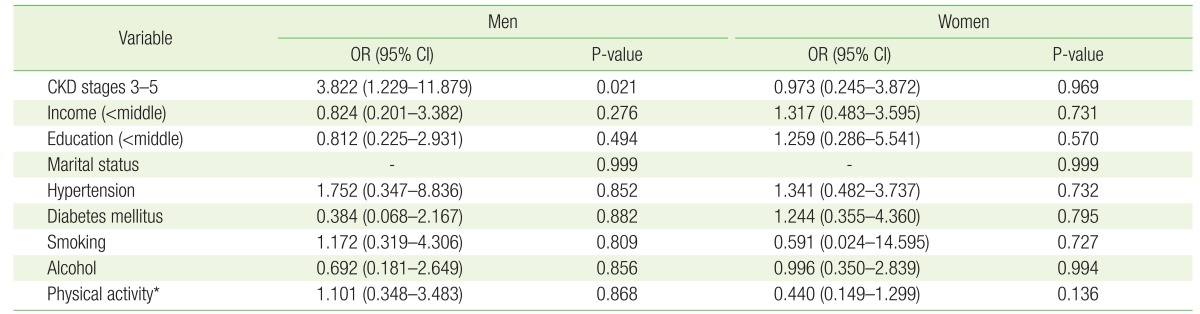
The prevalence of depression in older adults was 4.1% in men and 8.8% in women (P=0.004). The prevalence of depression did not differ according to CKD stage in women (normal eGFR and CKD stages 1 and 2 women, 41/474 [8.6%]) vs. CKD stages 3–5 women, 6/63 [9.5%]); however, the prevalence of depression in men with CKD stages 3–5 (8/83 [9.6%]) was significantly higher than in men with normal eGFR and CKD stage 1 and 2 (10/353 [2.8%], P=0.010). Multivariate logistic regression analysis showed that the odds ratio for depression in men with CKD stages 3–5 was 3.822 (95% confidence interval, 1.229 to 11.879) after adjusting for social status and chronic diseases (P=0.021).
Conclusion
The prevalence of depression was higher in elderly women than in men, while the prevalence of depression increased in elderly men with CKD stages 3–5 and was almost equal to that of women. Therefore, elderly men with progressive renal function impairment should be counseled and monitored for psychological problems.
INTRODUCTION
Chronic kidney disease (CKD) is highly prevalent and affects about 10% of the global population.123) The prevalence and incidence of renal replacement therapy has increased dramatically and the rate of increase is expected to be over 47% by 2020.4) Nearly one-third of community-dwelling adults >70 years old meet the criteria for CKD when it is defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2.5) Older adults also experience higher rates of eGFR <30 mL/min/1.73 m2 than do younger adults, in part due to higher rates of diabetes and hypertension.6) In addition to the traditional risk factors for initiating dialysis, such as cardiovascular disease, proteinuria, and diabetes, there is an increasing interest in psychosocial risk factors.7)
The elderly population in developing and developed countries worldwide is increasing gradually due to a prolonged life expectancy.8) At the same time, mental health problems in elderly adults have become a significant public policy concern. The prevalence of major depressive disorder in Korea ranges from 4.3% to 9.1%, and that of depressive symptoms ranges from 9.1% to 33.0%.9) In addition to its increasing prevalence in older adults, depression impacts older people's lives in a different manner than it affects younger people. Depression often occurs in elderly people with other illnesses and disabilities, and it is more prolonged. In addition, advancing age is often accompanied by a loss of social support systems due to the death of a spouse or siblings, retirement, or relocation of residence. Because of changing circumstances and because elderly people are expected to become less active, doctors and family members may miss the signs of depression.1011) As a result, effective treatment is often delayed, forcing many elderly people to struggle with depression unnecessarily.
The prevalence of depression in patients with CKD ranges from 7%–42%, which is higher than the prevalence seen in other chronic diseases and, as a result, is the most common psychological problem in patients with CKD.1213) Several studies in Western countries have investigated the effects of CKD, or end-stage renal disease (ESRD), on depression and found that these conditions are independently associated with a marked increase of both morbidity and mortality.14) Furthermore, patients with a clinical diagnosis of depression, who are undergoing long-term hemodialysis therapy, are twice as likely to die or require hospitalization within one year compared to those without depression.1516) A diagnosis of depression in patients with ESRD is independently associated with 30% increases in both cumulative hospital days and the number of hospitalizations, which, in turn, contributes to increased Medicare costs.17)
Therefore, our aim was to study the relationship between CKD and depression in elderly Korean adults and further evaluate sex-based differences using data from the Korea National Health and Nutrition Examination Survey (KNHANES).
METHODS
1. Study Participants and Database
We used secondary data from the 2013 KNHANES. The KNHANES is a nationwide representative cross-sectional survey designed to examine dietary habits, lifestyle behaviors, and the overall physical and mental health of the general Korean population. KNHANES was initiated by the Ministry of Health and Welfare in 1998. Annually, 10,000–12,000 individuals from 4,600 households are selected to represent Koreans aged ≥18 years using a multi-stage clustered and stratified random sampling method based on national census data.
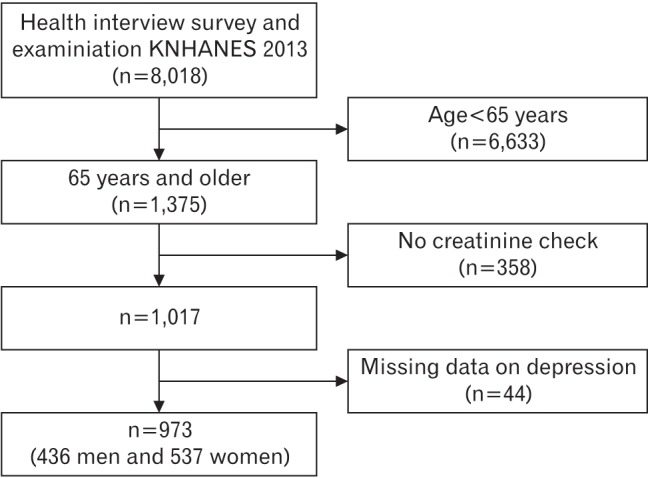
Of the 8,018 participants, we excluded subjects <65 years old (n=6,633), those with no serum creatinine (sCr) data (n=358), and those with missing data on depression (n=44). Thus, the final analysis included 973 subjects (436 men and 537 women) aged ≥65 years (Figure 1).
2. Chronic Kidney Disease Stages
eGFR, expressed in mL/min/1.73 m2, was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation:1819) for females with a sCr level ≤0.7 mg/dL, GFR=144×(sCr/0.7)-0.329×(0.993)age; for females with a sCr level >0.7 mg/dL, GFR=144×(sCr/0.7)-1.209× (0.993)age; for males with a sCr level ≤0.9 mg/dL, GFR=141×(sCr/0.9)-0.411×(0.993)age; and for males with a sCr level >0.9 mg/dL, GFR=141×(sCr/0.9)-1.209×(0.993)age. Participants with CKD (structural kidney or urinary abnormalities with or without a decreased GFR) were classified according to the system suggested in the Kidney Disease Outcome Quality Initiative of 2002.17) Due to the small sample sizes, subjects with kidney function stages 3–5 were combined into one group, leaving the following GFR groups for comparative analyses: normal eGFR and CKD stage 1, eGFR ≥90; CKD stage 2, eGFR 60–89.9; and CKD stages 3–5, eGFR <60 mL/min/1.73 m2.
3. Measurements and Definitions of General and Clinical Characteristics
For the present study, height, weight, and blood pressure (BP) measurements were obtained. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2) and all blood samples were collected in the morning following an overnight fast. Smoking status was categorized into two groups (‘yes’: those who were currently smoking and those who had smoked 100 or more cigarettes in their lifetime; and ‘no’: non-smokers, ex-smokers, and those who had smoked in the past but ceased), alcohol consumption status was defined as drinking more than once per month, and physical activity (PA) was determined using the Korean version of the International Physical Activity Questionnaire (IPAQ) by asking participants how often they engaged in exercise each week.20) Using the IPAQ, an average metabolic equivalent of task (MET) score was derived for each type of activity, and the following values were used for the analysis of IPAQ data: walking=3.3 METs, moderate PA=4.0 METs, and vigorous PA=8.0 METs. Using these values, four continuous scores were defined as walking MET-min/wk=3.3×walking minutes×walking days, moderate MET-min/wk=4.0×moderate-intensity activity minutes×moderate days, vigorous MET-min/wk=8.0×vigorous-intensity activity minutes×vigorous-intensity days, and total PA MET-min/wk=sum of walking+moderate+vigorous MET-min/wk scores.
Other medical conditions were defined by the KNHANES analysis guidelines as described below.21) Hypertension was defined as a systolic BP of ≥140 mm Hg, a diastolic BP of ≥90 mm Hg, or the current use of antihypertensive medications. Diabetes mellitus was defined as a plasma glucose level ≥126 mg/dL after at least 8 hours of fasting or diabetes treatment with either an oral hypoglycemic agent or insulin. Cardiovascular disease was defined as a history of angina pectoris or myocardial infarction.
Depression was defined as an answer of “yes” to the question of whether they had been diagnosed with depression by their doctor. In the KNHANES analysis, depressive symptoms were assessed with the following question: “In the past year, have you felt extremely sorrowful or despair for more than 2 weeks?”, which was answered with a “yes” or “no”. This questionnaire has been used to identify people with depressive mood since the start of the KNHANES in 1998. Additionally, the participants were asked whether they ever had a diagnosis of clinical depression confirmed by physicians, and those who answered “yes” reported their age at first diagnosis. The question regarding depressive symptoms has been widely used to assess participants and is considered to be a proxy for depressive disorder22) with a sensitivity of 86%, a specificity of 78%, and a positive and negative predictive value of 82% for the diagnosis of depression.23) To ensure an accurate analysis, only participants who were diagnosed with depression by physicians (the latter question) were included in the study.
4. Ethical Considerations
All patients in the KNHANES signed an informed consent form. Because this was a cross-sectional study based on KNHANES (http://knhanes.cdc.go.kr/knhanes/), ethical approval was not required.
5. Statistical Analysis
The statistical analysis was performed using IBM SPSS for Windows ver. 20.0 software (IBM Corp., Armonk, NY, USA). A complex sample analysis was performed based on an analysis plan file in which weights, stratification variables, and primary sampling units were designed. Continuous variables are expressed as mean±standard deviation and were compared using Student t-test. Categorical variables are expressed as proportions and were compared using the chi-square test. To identify the independent factors associated with depression, a complex sample logistic regression analysis was performed to identify associations between the stage of CKD and depression after controlling for covariates, such as income level, education level, marital status, hypertension, diabetes mellitus, smoking, alcohol, and PA. Due to skewed distributions, PA was log-transformed prior to the multivariate analysis. P-values <0.05 were considered to indicate statistical significance.
RESULTS
1. Clinical and Demographic Characteristics
The clinical characteristics of this sample of Koreans aged ≥65 years according to their depression status are provided in Table 1. The mean ages of those without and with depression were 71.2 and 73.1 years, respectively, in men, and 71.1 and 70.4 years, respectively, in women. A total of 65 of the 973 patients (6.7%) were diagnosed with depression. The prevalence of depression was more than two-fold higher in women than that in men (47/537 [8.8%] and 18/436 [4.1%], respectively). BMI, income level, educational status, marital status, smoking, alcohol, PA, chronic diseases such as hypertension and diabetes mellitus, and CKD stage were not related to depression in women. Alternatively, the prevalence of depression in men with CKD stages 3–5 was higher than that observed in men with a normal eGFR and CKD stages 1 and 2 (8/18 [44.4%] versus 75/418 [17.9%], respectively; P=0.013).
2. Prevalence of Depression According to Chronic Kidney Disease Stage in Men and Women
Figure 2 shows the prevalence of depression according to CKD stage. The prevalence rates of depression were 51/827 (6.2%) and 14/146 (9.6%), respectively, in the normal eGFR, and CKD stage 1 and 2 group and in the CKD stages 3–5 group for all patients (P=0.148). The prevalence rates of depression did not differ according to CKD stage in women (41/474 [8.6%] in the normal eGFR and CKD stages 1 and 2 group versus 6/63 [9.5%] the CKD stages 3–5 group; P=0.812) while the prevalence of depression in men with CKD stages 3–5 (8/83 [9.6%]) was significantly higher than that in men with normal eGFR and CKD stages 1 and 2 group (10/353 [2.8%], P=0.010). The prevalence of depression increased in elderly men as the kidney function worsened.

Prevalence of depression according to CKD stage. (A) The prevalence rates of depression were 51/827 (6.2%) and 14/146 (9.6%), respectively, in the normal eGFR and CKD stages 1 and 2 group and in the CKD stages 3–5 group for all patients (P=0.148). (B) The prevalence rates of depression were 10/353 (2.8%) and 8/83 (9.6%), respectively, in the normal eGFR and CKD stages 1 and 2 group and in the CKD stages 3–5 group in men (P=0.010). (C) The prevalence rates of depression were 41/474 (8.6%) and 6/63 (9.5%), respectively, in the normal eGFR and CKD stage 1 and 2 group and in the CKD stages 3–5 group in women (P=0.812). CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
3. Multivariate Logistic Regression Analysis of Chronic Kidney Disease and Depression
We conducted logistic analyses to investigate the association between CKD stage and depression. In men, the adjusted odds ratio (OR; 95% confidence interval [CI]) for depression in the CKD stages 3–5 group was 3.822 (1.229–11.879) compared to the normal eGFR and CKD stage 1 group (P=0.021) (Table 2). However, the OR for depression in the CKD stage 3–5 group was not significant in women (OR, 0.973; 95% CI, 0.245 to 3.872; P=0.969). CKD stages 3–5 were significantly associated with depression in elderly men.
DISCUSSION
This study provides a meaningful comparison of the prevalence of depression according to sex in elderly patients with CKD. The prevalence of depression was higher in women, regardless of CKD stage. However, the prevalence of depression increased in men as CKD stage increased (2.8% versus 9.6% in normal eGFR and CKD stages 1–2 versus CKD stages 3–5, respectively; P=0.010) (Figure 2). These results suggest that physicians should be aware of depression in men, as well as women patients, with CKD, particularly in elderly patients. Many studies have shown that major depression or depressive symptoms are associated with late-stage CKD, such as that reported in patients with ESRD on hemodialysis.2425) However, few data are available on the prevalence of depression in patients with earlier stages of CKD, before dialysis therapy is initiated. We investigated the relationship between all stages of CKD and depression, with a focus on elderly people.
It has been repeatedly shown that women are at greater risk for depression than men, and the female-to-male prevalence ratio for depression has been widely accepted to be approximately 2:1.8) Our results (4.1% versus 8.8% in men and women, respectively) for patients with all stages of CKD are consistent with this generally accepted ratio. However, some studies showed that this may be an artifact that reflects a greater tendency for women to seek help or over-report depressive symptoms compared with men.8) Furthermore, women may be more apt to dwell on their stress and turn transient negative emotions into severe depressive symptoms, whereas men tend to minimize their depressive mood and engage in more active behaviors.26) Interestingly, our results show that the prevalence of depression in men with impaired renal function (CKD stages 3–5) increased and was approximately equal to that seen in women (9.6% versus 9.5%, respectively). This result might reflect an association between more severe comorbidities and more depressive symptoms.
The present study also found an independent association between CKD stage and an increased risk of depression in elderly men with decreased kidney function, even after adjusting for other common comorbidities such as diabetes, hypertension, and lifestyle factors such as smoking, alcohol, and PA. It is possible that those in the lower eGFR category experienced symptoms more directly related to their kidney disease, including dyspnea, poor appetite, or fatigue, which might affect depression.
The potential mechanisms responsible for the association between depression and adverse clinical outcomes are not completely clear. One plausible mechanism is non-adherence to medical regimens, which has been observed in hemodialysis patients with depression who were shown to be less compliant with dietary and fluid restrictions.27) Some evidence suggests that depression may be associated with factors that could predispose patients to develop cardiac disease, such as lower heart rate variability,28) increased cortisol and norepinephrine excretion,29) and an altered immunological or stress response.30) Some studies show that patients with depression and ESRD have higher circulating levels of interleukin-6, tumor necrosis factor-α, and C-reactive protein, which are associated with poor outcomes.3132) Depression is also associated with poorer nutrition and leads to an upregulation of inflammatory mediators.33)
Even in the absence of a clear causal mechanism, several actions can be taken to improve clinical outcomes. Tsai et al.13) suggested that patients with CKD can be screened for depressive symptoms using the Beck Depression Inventory, particularly when a psychiatric consultation is unavailable or stigmatizing to the patient. This screening tool may help uncover a psychiatric illness and identify patients at high risk for depression. They also suggested that the care team begin preparation for ESRD earlier, and that referral to a nephrologist should be considered at an earlier stage, considering the possibility of rapid progression.13)
Our study has several limitations. First, the cross-sectional nature of the study made it impossible to assess any cause-and-effect relationships. Further prospective research is warranted to better assess the causal relationship between kidney function and depression in men. Second, eGFR was calculated using the CKD-EPI equation, which involves age, sex, and sCr level. However, sCr is affected by muscle mass. If the eGFR equation using cystatin-C rather than Cr had been used, the result would likely have been more accurate. Third, we did not separate patients with CKD stage 5 from those who were not undergoing hemodialysis. Finally, we could not exclude the effects of information bias because of the nature of the survey questionnaire. However, this study also had several strengths. Population-based nationwide representative data obtained by the KNHANES were used for this study. Therefore, a stratified multistage probability sampling method was used to obtain representative data of South Korean adults using a large sample size.
In conclusion, the prevalence of depression was higher in elderly women than in elderly men. However, the prevalence of depression in men with CKD stages 3–5 increased to almost the same prevalence as that seen in women. Therefore, elderly men with progressively impaired renal function should be counseled and monitored carefully. Further studies may be necessary to investigate the causal factors and methods to prevent and treat depression in elderly patients with kidney disease.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.