Association between Serum Uric Acid and Oxidative Stress in Korean Adults
Article information
Abstract
Background
Oxidative stress is implicated in the pathogenesis and development of lifestyle-related diseases. In the present study, we evaluated the correlation between the serum uric acid (UA) levels and oxidative status in Korean adults.
Methods
The subjects were 5,093 individuals (2,041 women and 3,052 men) who underwent a health checkup between June 2012 and December 2016. Oxidative stress levels (derivatives of reactive oxygen metabolites [d-ROMs]) and antioxidant potential (biological antioxidant potential [BAP]) were measured. Metabolic markers, including UA, were also examined.
Results
Higher serum UA levels were associated with decreased levels of d-ROMs (P<0.05). The UA levels were positively associated with BAP levels (P<0.001).
Conclusion
Serum UA is related to oxidative status, especially antioxidant capacity, in Korean adults; UA may play a role in antioxidant defense systems in humans.
INTRODUCTION
In recent years, oxidative stress has been identified as an important factor in the pathogenesis and development of lifestyle-related diseases. Especially, it is well-known that oxidative stress causes the progression and acceleration of atherosclerosis [1,2]. Moreover, oxidative stress caused by the increased production of reactive oxygen species (ROS) and/or decreased effectiveness of the antioxidant system is implicated in the pathogenesis of various disease entities, such as arteriosclerosis, malignant tumors, and autoimmune diseases [3-6].
Several antioxidant defense systems present in humans may act to correct a cellular redox imbalance; these include uric acid (UA), bilirubin, and albumin [7-9].
A potent natural antioxidant, UA has been claimed to exert preventive effects against the development of age-related neurodegenerative diseases [10]. It protects against vascular damage mediated by oxidative stress following ischemia/reperfusion in rats [11].
In this study, we investigated the association between UA and the derivatives of reactive oxygen metabolites (d-ROMs), as an index of the products of ROS, and biological antioxidant potential (BAP), as an index of the antioxidant potential [1].
METHODS
1. Subjects
The subjects were 5,093 individuals (2,041 women and 3,052 men) who underwent a health checkup between June 2012 and December 2016. We excluded eight out of 5,101 people due to unavailability of data on serum UA. The majority of these subjects visited the clinic for regular health checkups, and therefore, did not have any serious health problems. The demographic information (age, sex, smoking, and drinking), medical history, and medication history of the subjects were investigated and recorded. Smoking status was categorized as non-smoking or currently smoking. Subjects who had been smoking cigarettes regularly 1 year before the survey were considered current smokers. Alcohol consumption was divided into the following categories: nondrinkers, current drinkers. The current drinker category was used for subjects who drank alcohol 2 times a week.
2. Data Measurement
Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) [12]. Blood pressure was measured by an automated sphygmomanometer after approximately 10 minutes of rest. Hypertension was defined as a systolic blood pressure (SBP) ≥140 mm Hg or a diastolic blood pressure (DBP) ≥90 mm Hg or if the participant was using antihypertensive medication. Diabetes was defined as the hemoglobin A1c level greater than 6.5% or use of hypoglycemic medications. Dyslipidemia was defined as a low-density lipoprotein (LDL) cholesterol level of 160 mg/dL or greater, or self-reported use of cholesterol-lowering supplements.
After overnight fasting, a venous blood sample was obtained in the morning. Each subject fasted for more than 10 hours before blood collection. Serum UA levels were assayed using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan).
Both the oxidative stress level and antioxidant properties in serum were determined using an automated method. The oxidative stress level was measured via a d-ROM test (Diacron Srl, Grosseto, Italy), in which the amount of organic hydroperoxide converted into radicals that oxidize N,N-diethyl-p-phenylenediamine hydroperoxide was measured [3]. The results of the d-ROM test are expressed in an arbitrary unit called Carratelli unit (U.CARR) [13-15].
In the BAP test (Diacron Srl), antioxidant potential was measured based on the capacity of the plasma sample to reduce ferric (Fe 3+) ions to ferrous (Fe 2+) ions [1,16,17]. The results are expressed as mmol/L [13].
3. Statistical Analysis
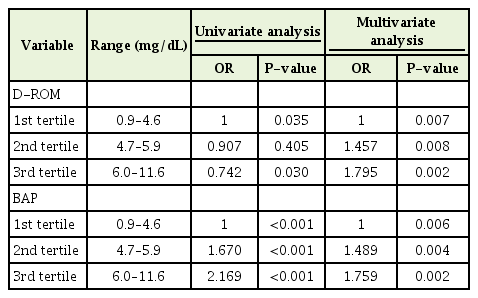
The results of grouped data were expressed as the means±standard deviation (SD). Analysis of variance trend analysis was adapted. Distribution of d-ROMs and BAP values was left-skewed. Thus, a natural log-transformation was applied. We performed univariate analysis according to serum UA levels, and multivariate analysis after adjusting for age, sex, BMI, smoking status and alcohol drinking, SBP, total glyceride (TG), high-density lipoprotein (HDL), and LDL. Data analysis was conducted using IBM SPSS Statistics ver. 21.0 software (IBM Corp., Armonk, NY, USA). A P-value <0.05 was considered statistically significant.
RESULTS
1. Baseline Characteristics
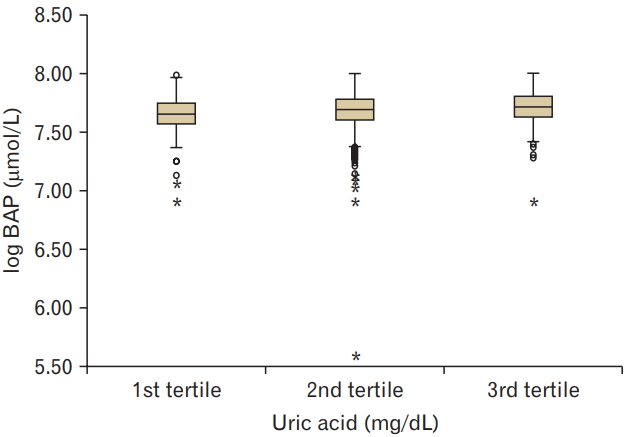
Clinical and biochemical data (mean±SD, or subject numbers [percentage]) were categorized by serum UA values into three groups. Ranges of the 1st, 2nd, and 3rd tertile of serum UA values were 0.9–4.6, 4.7–5.9, and 6.0–11.6 mg/dL, respectively. The mean age of the subjects enrolled was 50.7±10.4 years, and 59.9% (n=3,052) of them were men. The results showed that smoking and alcohol consumption was associated with increased serum UA levels. The mean d-ROM value was 346.6±1.2 U.CARR and BAP value was 2,181.6±1.1 mmol/L. Higher serum UA levels were associated with decreased levels of d-ROM (P for trend <0.05) and increased levels of BAP (P for trend <0.001) (Figures 1, 2).
On univariate analysis, serum UA levels were found to be positively correlated with age, BMI, and blood pressure. Clinical and laboratory characteristics of the subjects, according to their serum UA levels, are listed in Table 1.
2. Relationships of Derivatives of Reactive Oxygen Metabolites and Biological Antioxidant Potential with Uric Acid
There were significant differences according to serum UA levels. These associations remained statistically significant after adjusting for age, sex, BMI, smoking status and alcohol consumption, SBP, TG, HDL, and LDL (Table 2).
DISCUSSION
This study shows that higher tertiles of serum UA levels were related to decreased levels of d-ROM and increased levels of BAP in subjects. Multiple regression analysis showed that the levels of d-ROM and BAP were closely related to serum UA levels, independently of age, sex, BMI, blood pressure, LDL cholesterol, HDL cholesterol, triglyceride, smoking, and alcohol consumption. These findings suggest a positive correlation between the levels of serum UA and BAP.
Previous studies have demonstrated that circulating UA may exert either anti- or pro-oxidant activity in-vivo [18,19], but anti- and pro-oxidant markers have not previously been simultaneously measured in a large population and their relation to circulating UA levels has not been assessed, as in the present study. We demonstrate that serum UA has antioxidant capacity.
Fukui et al. [1] showed that UA was positively associated with BAP. As a factor that influences BAP, the serum UA level was selected; UA is recognized as the primary defense against oxidative stress in extracellular fluids [9]. On the other hand, A study conducted in Japan showed that higher serum UA levels were associated with increased levels of d-ROMs in both sexes [7]. The difference in some observations might be attributed to variations in sample size, the statistical methods used, and the consideration or non-consideration of albuminuric status [7].
It is well-known that high plasma UA level is strongly associated with peripheral, carotid, and coronary vascular diseases; development of stroke; and vascular dementia [9,17,20,21]. The relationship of UA with cardiovascular events is particularly strong, especially in patients at high risk for heart diseases. Whether UA has a causal relationship in these conditions remains to be determined.
Oxidative stress may be associated with not only lifestyle-related diseases but also various other diseases. Therefore, our results suggest the significance of oxidative stress measurement in a large number of subjects. For a better understanding of these double-sided actions of UA, future studies should clarify whether the harmful effects of UA are caused by its pro-oxidant properties, and conversely, whether the beneficial effects of UA are attributable to its antioxidant potential.
A limitation of our study is that we analyzed data from participants who voluntarily visited a health promotion center; this group might not be representative of the general population.
In conclusion, in the present cross-sectional study, serum UA level was found to be closely associated with d-ROM and BAP levels in Korean adults. Serum UA levels are related to oxidative status, especially antioxidant capacity. Therefore, UA may play a role in the antioxidant defense systems in humans.
Notes
No potential conflict of interest relevant to this article was reported.