Association of Lung Function with Serum 25-Hydroxyvitamin D Level according to the Presence of Past Pulmonary Tuberculosis in Korean Adults
Article information
Abstract
Background
Vitamin D deficiency is associated with an increased risk of pulmonary tuberculosis (PTB) infection and the treatment outcome. The aim of this study was to examine the relationship between the serum 25-hydroxyvitamin D (25[OH]D) level and lung function in Korean adults according to whether or not there is a history of PTB.
Methods
The data for subjects aged 19 years or older from the Korea National Health and Nutrition Examination Survey 2008–2012 who underwent spirometry, chest radiography, and serum 25(OH)D level measurement were analyzed.
Results
Evidence of past PTB infection was found in 1,482 (9.6%) of 15,516 subjects. The serum 25(OH)D level was lower in the group with past PTB than in the non-PTB group (P=0.013). Respiratory dysfunction was more common in the past PTB group than in the non-PTB group (restrictive pattern, 14.0% vs. 9.6%; obstructive pattern, 29.6% vs. 8.2%; both P<0.001). After adjusting for age, sex, height, and season, the mean difference in forced expiratory volume in 1 second (FEV1) between the highest and lowest quartiles of 25(OH)D was 100.2 mL (standard error=49.3 mL, P for trend=0.049) in the past PTB group and 34.7 mL (standard error=13.6 mL, P=0.009) in the non-PTB group.
Conclusion
FEV1 tended to increase as the vitamin D quartile increased in both study groups. This relationship was more pronounced in subjects with a history of PTB. A higher serum 25(OH)D level might be beneficial in preserving lung function after PTB infection.
INTRODUCTION
The incidence of pulmonary tuberculosis (PTB) in South Korea continues to be the highest among the Organization for Economic Cooperation and Development countries [1]. In 2016 alone, there were 39,245 patients with PTB undergoing treatment and 30,892 newly diagnosed patients in South Korea. According to the Korea Tuberculosis report from the Korea Centers for Disease Control and Prevention (KCDC), 2,209 individuals died from PTB in 2015. Although the number of deaths is decreasing, PTB remains an important public health problem in South Korea [2].
Vitamin D deficiency is associated with an increased risk of PTB infection [3], and an adequate vitamin D level can lower the risk of relapse of TB [4]. It has been reported that serum 25-hydroxyvitamin D (25[OH]D) levels in persons who have recovered from PTB are consistently lower than the levels in those without a history of PTB [5]. Vitamin D plays an important role in the immune response to Mycobacterium tuberculosis by promoting both the formation of phagolysosomes and production of antimicrobial peptides with bactericidal activity and immune-regulating functions [6].
Nationwide population studies have shown that there is a positive relationship between the serum 25(OH)D level and lung function [7-9]. This association was found to be more prominent in subjects with a history of PTB than in those with asthma or chronic obstructive pulmonary disease (COPD) [9]. These observations suggest that individuals with vitamin D deficiency are more vulnerable to PTB-induced lung damage. PTB causes structural damage and compromises the vascular system, resulting in permanent scarring that may lead to decreased lung function. Even persons with spontaneously healed PTB and TB scarring on chest radiographs may have impaired lung function [10]. Therefore, individuals with a history of past PTB or TB scars on chest radiographs were included in past PTB group in this study, the aim of which was to examine the relationship between the serum 25(OH)D level and lung function in Korean adults according to the presence of past PTB.
METHODS
1. Study Population and Database
Data were obtained from the Korea National Health and Nutrition Examinations Survey (KNHANES) 2008–2012 conducted by the KCDC. The KNHANES is a cross-sectional survey that is performed every year and is designed to examine the health and nutritional status of the non-institutionalized Korean population. The survey consists of a health interview and nutrition questionnaire. KNHANES uses a multistage cluster probability sampling design to ensure independent and homogeneous sampling for each year in addition to nationally representative sampling.
A total of 17,135 participants aged 19 years or older in KNHANES underwent spirometry. In this study, individuals without a serum 25(OH)D level measurement (n=789) and those with an estimated glomerular filtration rate <30 mL/min (n=28) were excluded, as were those with findings of active PTB or pulmonary disease other than PTB (n=802). Finally, 15,516 individuals were eligible for this study.
All study protocols were approved by the KCDC Institutional Review Board (IRB approval no., 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, and 2012-01EXP-01-2C). All subjects volunteered and provided written informed consent prior to participating in this study.
2. Measurement of Serum 25-Hydroxyvitamin D Level
A blood sample was obtained from each study subject, refrigerated, transported to the Central Testing Institute, and analyzed within 24 hours. The serum 25(OH)D concentration was measured using a 25(OH)D radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA) with a gamma-counter (1470 Wizard; PerkinElmer Life Sciences Inc., Waltham, MA, USA). To interpret lung function, the serum 25(OH)D levels were divided into four quartiles: Q1 (<15.1 ng/mL), Q2 (15.1–19.1 ng/mL), Q3 (19.1–23.9 ng/mL), and Q4 (≥23.9 ng/mL) for men, and Q1 (<12.7 ng/mL), Q2 (12.7–16.1 ng/mL), Q3 (16.1–20.5 ng/mL), and Q4 ≥20.5 ng/mL for women.
3. Pulmonary Function Tests
Pulmonary function tests were performed using a dry rolling seal spirometer (Model 2130; Sensor-Medics, Yorba Linda, CA, USA). Spirometry was conducted by pulmonary laboratory technicians. The results were transferred to an Internet review center for processing. The data were carefully examined and compared against criteria metrics for acceptability, reproducibility, and quality control. Airflow obstruction was defined as a forced expiratory volume 1 second (FEV1)/forced vital capacity (FVC) <70% or the lower limit of normal. Restrictive airflow was defined as a reduction in FVC to <80% of the predicted value.
4. Chest Radiographs
Chest radiographs were taken using a DigiRAD-PG machine (Digirad Corp., Suwanee, GA, USA) installed on an examination vehicle. The presence of non-calcified nodules with distinct margins, calcified nodules, and discrete linear or reticular fibrotic scars with or without calcification were interpreted as PTB scarring. Each chest radiograph was interpreted by two radiologists working independently for the presence of lung disease. Individual readings were compared weekly. Chest radiographs demonstrating PTB-related lesions were re-interpreted by six radiology specialists to confirm the results.
5. Definition of Past Pulmonary Tuberculosis and Other Variables
Subjects with a self-reported history of physician-diagnosed PTB or TB scarring on chest radiography were defined as having evidence of past PTB. Household income was divided into high/upper middle and lower middle/low. Heavy drinking was defined as more than seven drinks (for men) or five drinks (for women) on a single day at least twice a week. Regular exercise was defined as engaging in moderate (30 minutes on each occasion at least 3 times per week) or vigorous (20 minutes on each occasion at least 3 times per week) exercise on a regular basis. Education was divided into university/high school graduate (10th grade or higher) and middle school graduate (9th grade and lower).
South Korea is located in a temperate region. Therefore, seasonal variables were adjusted for serum vitamin D levels with four distinct seasons: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February).
6. Statistical Analysis
A sample weight was assigned for participating individuals to produce an unbiased national estimate. Sampling weights were constructed to account for the complex survey design, non-response to the survey, and post-stratification. Continuous variables are expressed as the mean and standard error and categorical variables as the number and percentage. The baseline characteristics, lung function, and serum 25(OH)D levels in the past PTB and non-PTB groups were analyzed using Student’s t-test or the chi-square test. The association of 25(OH)D with FEV1 or FVC was modeled by multiple linear regression with the Bonferroni post-hoc test. Deviation of linearity across the quartiles of 25(OH)D levels was assessed by the likelihood test for the quadratic term. All statistical analyses were performed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA). A P-value <0.05 was considered statistically significant.
RESULTS
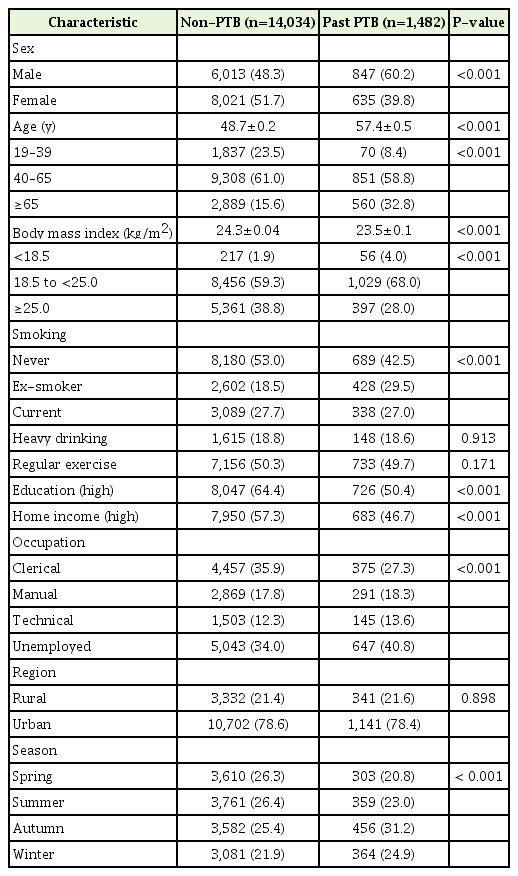
Evidence of past PTB infection was found in 1,482 (9.6%) of 15,516 subjects. The baseline characteristics of these subjects are summarized in Table 1. The mean age of the subjects in the past PTB group was higher than that in the non-PTB group (57.4 versus 48.7 years, P<0.001) and there were more men in the past PTB group than in the non-PTB group (60.2% versus 48.3%). The percentage of never-smokers was higher in the non-PTB group and the percentage of eversmokers was higher in the past PTB group.
The past PTB group had a lower FEV1, FVC, and FEV1/FVC ratio (85.3%, 89.3%, and 0.73, respectively) than the non-PTB group (93.2%, 93.7%, and 0.80) (Table 2). The proportions of restrictive pattern and obstructive pattern were higher in the past PTB group than in the non-PTB group (14.0% versus 9.6% and 29.6% versus 8.2%, respectively; both P<0.001) (Figure 1).

Proportion of those with respiratory dysfunction according to history of PTB. The proportion of restrictive pattern and obstructive pattern in the past PTB group was higher than that in the non-PTB group (14.0% vs. 9.6% in restrictive pattern and 29.6% vs. 8.2% in obstructive pattern, both P<0.001). PTB, pulmonary tuberculosis.
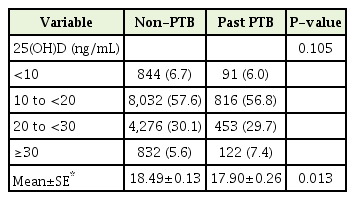
According to the definition of vitamin D deficiency (<20 ng/mL) [11,12], 92.5% in the past PTB group and 94.4% in the non-PTB group were categorized as having an inadequate 25(OH)D level (<30 ng/mL) (Table 3). There was no significant difference in the distribution of 25(OH)D levels between the two groups. After adjusting for age, sex, season, and body mass index, the 25(OH)D level was lower in the past PTB group than in the non-PTB group.
The mean difference in FEV1 increased in the crude group with increasing 25(OH)D quartile (Q2, Q3, and Q4: 54, 58, and 123 mL in the past PTB group, P for trend=0.021; 20, 26, and 35 mL in the non-PTB group, P for trend=0.007). The difference between the top and bottom quartiles of FEV1 was greater in the past PTB group than in the non-PTB group.
After adjusting for age, sex, height, and season, the FEV1 increased in both study groups as the 25(OH)D quartile increased (Table 4). With further adjustment for smoking status, exercise, body mass index, occupation, and region, there was a significant increase in FEV1 in models 2 and 3 (Table 4). In the non-PTB group, there was a positive association between FVC and the serum 25(OH)D quartiles in models 1 and 2 (Table 5).

Difference in forced expiratory volume in 1 second by serum 25(OH)D quartile according to evidence of PTB
DISCUSSION
In this study, individuals with past PTB had worse pulmonary function (FEV1, FVC, FEV1/FVC ratio) and lower 25(OH)D levels than those without a history of PTB. FEV1 tended to increase with increasing 25(OH)D quartile. The difference in the 25(OH)D level between the highest and lowest quartiles for FEV1 was greater in the group with past PTB than in the group without past Tb (123 mL versus 35 mL). This finding indicates that lung function was more influenced by the serum 25(OH)D level in the past PTB group than in the non-PTB group.
A positive relationship between the serum 25(OH)D level and lung function has been reported by several nationwide population studies. According to a National Health and Nutrition Examination Survey in the United States, the mean difference in FEV1 between the top and bottom quintiles of serum 25(OH)D was 126 mL (P<0.0001) [9]. Cross-sectional data obtained from a nationwide cohort in the United Kingdom revealed a linear relationship between the 25(OH)D level and lung function in that the FEV1 value was 8 mL higher in volume for each 4 ng/mL of increase in the 25(OH)D level [7]. A positive relationship between the serum 25(OH)D level and lung function has also been confirmed in the Korean population [9].
Vitamin D deficiency is a risk factor for PTB because of its essential role in the immune system [13]. Vitamin D appears to play a part in innate immunity via the nuclear vitamin D receptor (VDR). Activation of the VDR induces production of cathelicidin antimicrobial peptide from macrophages and epithelial cells, thereby enhancing autophagy in monocytes in response to the antimicrobial effect of cathelicidin [14]. Vitamin D also plays a role in acquired immunity by inhibiting induction of apoptosis in activated B-cells and modulating proliferation of T lymphocytes [15]. The number of candidate VDR polymorphisms has been found to be associated with susceptibility to PTB [16]. Vitamin D deficiency is associated with decreased lung function [17-19]. Vitamin D also inhibits formation of matrix metalloproteinase-9 (MMP-9) [20], thereby influencing degradation of tissue remodeling in the lung parenchyma. In addition, vitamin D suppresses inflammation in the airways by reducing the production of inflammatory cytokines and chemokines by epithelial cells [21].
The results of the present study are similar to those of a meta-analysis that found the 25(OH)D level to be lower in individuals with PTB [3]. Moreover, the vitamin D level has been reported to remain consistently low even after treatment of PTB [5]. Patients with vitamin D insufficiency (<30 ng/mL) tend to have a higher likelihood of recurrence of PTB [4], which results in more impairment of lung function because of the residual damage to lung tissue [22]. Furthermore, vitamin D deficiency plays a role in the development of COPD with increased MMP-9 level [23], thus increasing the risk of COPD and decreasing lung function in the past PTB group with low 25(OH)D level. In the population-based Burden of Obstructive Lung Disease study, a history of PTB was associated with both airflow obstruction and spirometric restriction [24].
Randomized clinical trials have yielded inconsistent results with regard to the efficacy of vitamin D supplementation for PTB. In an Indonesian study, negative sputum conversion with radiographic improvement was achieved in 100% of 67 patients supplemented with vitamin D 0.25 mg/d, whereas only 78.7% of those who received placebo showed negative sputum conversion [25]. However, a clinical trial in India found no significant difference in sputum culture conversion time between a vitamin D supplementation group and a placebo group [26]. The reasons for these inconsistent results are unclear. One possible explanation is that the optimal vitamin D concentration for respiratory health is as yet unknown. Another explanation may lie in the various genetic polymorphisms of the VDR. One randomized clinical trial of vitamin D supplementation found clinical and radiographic improvement in patients with TB and vitamin D deficiency [27]; however, another study found that the sputum culture conversion time was only shortened in individuals with the tt genotype of TaqI VDR polymorphism [28]. Further studies are needed to identify the benefits of vitamin D supplementation according to VDR polymorphism in subjects with PTB.
A previous Korean report demonstrated that a significant number of people who did not report a history of PTB had evidence of TB scars on chest radiographs (i.e., spontaneously healed PTB) [29]. A prospective cohort study that investigated subjects with spontaneously healed PTB and normal subjects over a 10-year period found a greater decrease in predicted FEV1 in those with spontaneously healed PTB [10]. Excluding individuals with TB lesions on chest radiography may underestimate the association between TB and respiratory dysfunction [30].
This research has several limitations. First, the study had a cross-sectional design, so reverse causality as an explanation for the observed association could not be ruled out. Second, the severity of PTB-induced lung damage and the presence of recurrence could not be determined because this information is not available in KHANES. Third, the self-reported history of the study participants did not contain information on vitamin D supplementation or medication that might affect the serum 25(OH)D level.
In conclusion, we have found a tendency for FEV1 to increase with increasing vitamin D level regardless of whether or not there is a history of PTB. In this study, the increase in FEV1 was greater for the higher vitamin D quartiles, especially Q4 in the group with past PTB. These results suggest that a higher 25(OH)D level may have a beneficial role in preserving lung function after PTB infection. Further studies are needed to identify the optimal vitamin D concentration for respiratory health.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The statistical analysis in this study was supported by a grant (HI14C1731) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Korea.