Clinical Characteristics Associated with Electrocardiographic Left Ventricular Hypertrophy in Clinical Normotensives without a History of Hypertension: a Cross-Sectional Study
Article information
Abstract
Background
This study evaluated factors independently associated with electrocardiographic left ventricular hypertrophy (ECG-LVH) in subjects who were normotensive on clinical measurement and had no prior history of hypertension.
Methods
This cross-sectional study analyzed cases and controls in the Comprehensive Medical Examination Center of Hallym University Sacred Heart Hospital. Eligible case participants presented ECG-LVH according to the Sokolow-Lyon or Cornell criteria, were normotensive on clinical measurement, and had never received a diagnosis of hypertension. The control group comprised subjects with normal sinus rhythm who were normotensive on clinical measurement with no history of hypertension.
Results
A multiple logistic regression model showed male sex, age and systolic blood pressure to be positively related to the presence of ECG-LVH. A positive relation of smoking and regular exercise; an inverse relation of pulse rate to the presence of ECG-LVH were found only in men. An inverse relation of uric acid level was found only in women. Detailed analyses of relatively healthy and young men according to whether or not to exercise regularly showed that positive relations of age and systolic blood pressure; an inverse relation of obesity to the presence of ECG-LVH were apparent in the non-regular exercise group but not in the regular exercise group. In the regular exercise group, only pulse rate showed significant (inverse) association with the presence of ECG-LVH.
Conclusion
The varying risk factor profiles associated with ECG-LVH according to sex and the participation in regular exercise may help to elucidate the ECG-LVH in clinical normotensives with no prior history of hypertension.
INTRODUCTION
The presence of left ventricular hypertrophy (LVH) is associated with an increased risk of sequelae from cardiovascular disease [1]. Reports have documented risk factors for the development of LVH such as age, elevated blood pressure (BP), obesity, stature, and glucose intolerance. Cardiac valve disease and chronic heart disease also cause LVH [2]. Regression or prevention of LVH are known to be associated with the reversal of an adverse cardiovascular prognosis [1]; thus, understanding of the development of LVH and suggesting individualized strategies are important. In this regard, LVH in hypertensive patients is relatively easy to interpret as it is evidence of hypertensive target organ damage [3], but the clinical significance or interpretation of LVH found incidentally in normotensives is uncertain [4]. Furthermore, to investigate determinants of LVH, most studies [1,3,5-7], except for a few [4,8], have been performed in hypertensive patient populations.
On the other hand, although LVH can be diagnosed either by electrocardiography (ECG) or by echocardiography (ECHO), owing to its low cost and non-invasiveness, ECG is more available in primary health care assessment and for the health screening of asymptomatic visitors. Thus, the issue of a correct interpretation of LVH found incidentally in normotensives will arise mainly after the observation of electrocardiographic LVH (ECG-LVH) rather than by echocardiographic LVH (ECHO-LVH).
Therefore, we investigated factors independently associated with ECG-LVH determined by commonly used Sokolow-Lyon or Cornell criteria [9] in participants who were normotensives on clinical measurement and who had never received a diagnosis of hypertension, in the context of exploring the clinical implications of ECG-LVH in such population.
METHODS
1. Subjects
A cross-sectional study was carried out at the Comprehensive Medical Examination Center of Hallym University Sacred Heart Hospital. Initially 11,713 participants visited the center from 2013 to 2014 as asymptomatic health screening subjects. Among the 11,713 participants, we subsequently excluded individuals whose ECG findings were neither ECG-LVH without other findings including ST-T changes/arrhythmias nor normal sinus rhythm (NSR) (n=5,827); individuals who were 19 years old or younger (n=45); individuals whose information on BP was missing (n=130); individuals with systolic blood pressure (SBP) ≥140/90 mm Hg or who had a prior diagnosis of hypertension (n=3,128). A total of 1,203 men and 1,380 women were ultimately included in this study (Figure 1) and divided into two groups (cases and controls). Eligible case participants included individuals with ECG-LVH with no other ECG findings including ST-T changes and arrhythmias. The case participants were those with both SBP <140 mm Hg and diastolic BP (DBP) <90 mm Hg on clinical measurement and had no previous history of a diagnosis of hypertension. The control group consisted of subjects with NSR who were normotensives on clinical measurement with no history of hypertension. Of those, the number of subjects with information on clinical variables is shown in Table 1. The subjects provided informed consent before participating in this study. All study protocols were approved by the Hallym University Sacred Heart Hospital institutional review board (IRB approval no., 2016-I083).

Flowchart describing the selection of the study population. ECG, electrocardiography; ECG-LVH, electrocardiographic left ventricular hypertrophy; NSR, normal sinus rhythm; BP, blood pressure.
2. Measurements
Standard 12-lead ECGs were recorded for all subjects at 25 mm/s and 1-mV/cm calibration. ECGs were retrieved from the ECG database (GE Muse; GE Healthcare, Waukesha, WI, USA), and were all reviewed by trained administrative staff and physicians. The ECG-LVH was determined according to the Sokolow-Lyon criteria (SV1+RV5 or RV6 ≥3.5 mV) [10] and/or by the Cornell criteria (SV3+RaVL ≥2.8 mV in men and ≥2.0 mV in women) [11]. BP and pulse rate (PR) were measured using a DINAMAP DPC100X-EN automated BP monitor (GE Medical System Information Technologies, Milwaukee, WI, USA). If the automatically measured BP was beyond the normal range, a trained nurse manually measured the BP with a sphygmomanometer, and the manually measured level was used for analysis.
We obtained data describing medical history and lifestyle from structured self-questionnaires. Education level was categorized into: <college education or ≥college education. Monthly household income was categorized into: <2,000,000 won/mo or ≥2,000,000 won/mo [12]. Marital status was defined as single or married. The widowed and divorced were considered as single. Smoking status was classified into three groups: never-smokers, ex-smokers, or current smokers. Regular exercise was defined as at least 150 minutes of moderate-intensity aerobic physical activity weekly or at least 75 minutes of vigorous-intensity aerobic physical activity weekly [13]. Men up to 65 years of age who consumed >4 standard drinks in a day (or >14 per week) and women and adults over 65 years who consumed >3 standard drinks per day (or >7 per week) were classified as ‘problem drinkers’ according to the National Institute of Alcohol Abuse and Alcoholism criteria [14]. Family histories of stroke, cardiac disease including ischemic heart disease, hypertension, diabetes, etc. were collected. The diagnosis histories of stroke, cardiac disease including ischemic heart disease, diabetes, dyslipidemia, tuberculosis, etc., were also collected. In addition, female participants completed a questionnaire on gynecological history. Menopause was defined as the stopping of the menstrual cycles. Postmenopausal women who had used hormone replacement therapy (HRT) to alleviate climacteric symptoms were classified as ‘ever users of HRT.’
Height, weight, and waist circumference were measured. Weight and height were measured by trained nurses using calibrated equipment. The body mass index (BMI) was calculated as weight (kg)/height (m)2. Waist circumference was measured to the nearest 0.1 cm during exhalation, using a measuring tape at the horizontal plane midway between the inferior costal margin and iliac crest at the midaxillary line. A participant with a BMI of ≥25 kg/m2 was considered ‘obese.’ [15]
Blood samples were obtained for routine blood chemistry tests. Samples were collected from the brachial vein after the subjects had fasted for at least 12 hours. The hemoglobin level was checked by cytochemical reaction using ADVIA 2120i (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). The hemoglobin A1c level was checked by high performance liquid chromatography using Variant II Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA). The fasting blood glucose (FBS) level was measured using the hexokinase method; the levels of total cholesterol and low-density lipoprotein were measured via enzymatic assay; high-density lipoprotein level was measured using the polyethylene glycol method; the triglyceride level was measured using enzymatic with glycerol blank method; the levels of aspartate aminotransferase (AST) and alanine transaminase were measured via UV with P5P method; uric acid was measured using the uricase method (Auto-analyzer Model 7600 II; Hitachi, Tokyo, Japan). The high-sensitivity C-reactive protein level was checked by turbidimetry using the Toshiba TBA 120FR Chemistry analyzer (Toshiba, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was determined by the Modification of Diet in Renal Disease standardized serum creatinine (eGFR [mL/min/1.73 m2]=175×serum creatinine [mg/dL]−1.154×age−0.203×0.742 [if female]).
3. Statistical Analysis
All data were analyzed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). Because sex differences were expected [3], all analyses were stratified by sex unless specified. The assumption of normality of the data was tested using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. The independent sample t-test and the Mann–Whitney U-test were used to analyze continuous variables. Categorical variables were analyzed by the chi-square test or Fisher’s exact test. Univariable and multivariable analyses were performed by means of binary logistic regression. Most parameters significant at P<0.05 in the univariable model were entered into the multivariable model. We also adjusted the multivariable model for variables that were not significant but could have a significant association with ECG-LVH. All tests were two sided, and the level of significance was set at P<0.05.
In the detailed analysis, we examined factors associated with ECG-LVH comparing those who did regular exercise (regular exercise group) with those who did not (non-regular exercise group). Subjects <60 years of age without a history of stroke, cardiac disease including ischemic heart disease, diabetes, and dyslipidemia as well as hypertension were investigated further in the regular exercise-stratified analyses.
RESULTS
1. Characteristics of Study Participants
The baseline characteristics of men and women with ECG-LVH or NSR are presented in Table 1. The prevalence of ECG-LVH was 2.9% (5.0% in men, 1.2% in women) in participants who were normotensives on clinical measurement and never had a diagnosis of hypertension. In both sexes, participants with ECG-LVH had a significantly higher SBP (men: median [interquartile range], 123.0 [117.0–130.0] mm Hg versus 119.0 [111.0–128.0] mm Hg; P=0.004; women: median [interquartile range], 117.5 [106.8–129.5] mm Hg versus 109.0 [102.0–118.0] mm Hg; P=0.007) than those with NSR. Men with ECG-LVH were more likely to do regular exercise (43.3% versus 21.1%, P=0.004) and to have lower PR (median [interquartile range]: 66.0 [62.0–72.8] versus 70.0 [65.0–76.0], P=0.014) than those with NSR. Women with ECG-LVH were more likely to be older (median [interquartile range]: 52.0 [44.8–55.8] versus 42.0 [37.0–49.0], P<0.001), have a higher FBS (median [interquartile range]: 90.0 [85.0–99.5] versus 86.0 [82.0–92.0], P=0.047) and higher hemoglobin level (median [interquartile range]: 13.7 [13.4–14.1] versus 13.3 [12.7–13.8], P=0.007), to have a history of dyslipidemia (18.8% versus 3.7%, P=0.022), and to be post-menopausal (69.2% versus 17.6%, P<0.001) than those with NSR.
2. Factors Associated with Electrocardiographic Left Ventricular Hypertrophy
In univariable logistic regression analysis, the factors associated with increased risk of ECG-LVH were as follows (Supplementary Table 1): higher SBP in both sexes; smoking (ex-smoker versus never smoker) and regular exercise in men; older age, a history of stroke, a history of dyslipidemia, higher hemoglobin and AST levels, and menopause in women. Whereas higher PR in men, monthly household income (≥2,000,000 won/mo versus <2,000,000 won/mo) and higher uric acid level in women were significantly associated with a decreased risk of ECG-LVH. Meanwhile, obesity was not a significant variable at the significance level P<0.05 in the univariable analysis. Because previous studies have reported significant relationships found between obesity and ECG-LVH [1,6], we also included this variable in the multivariable logistic regression model.
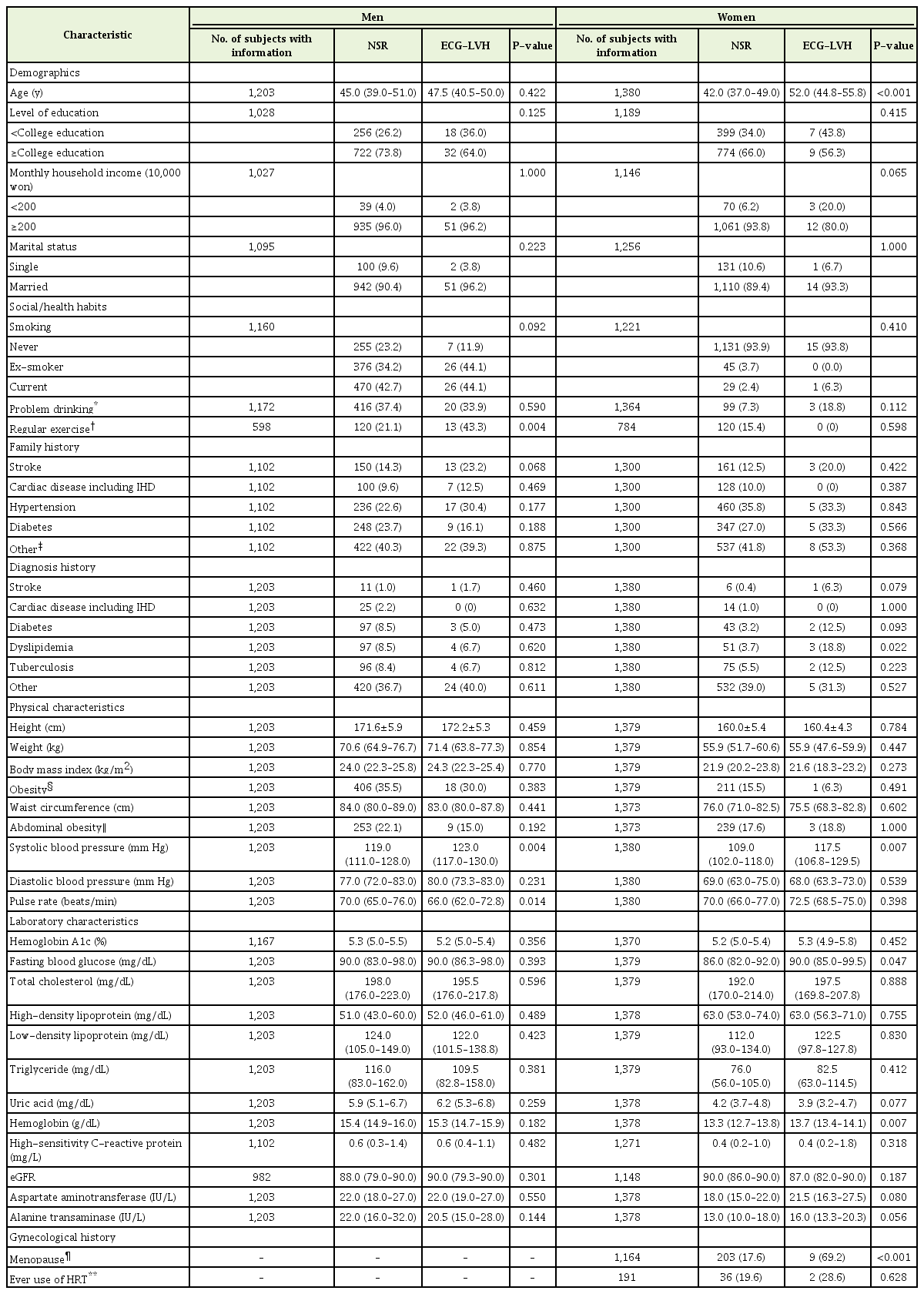
A sex-combined multivariable logistic analysis showed male sex (adjusted odds ratios [OR], 3.39; 95% confidence interval [CI], 1.90–6.03), age (adjusted OR, 1.03; 95% CI, 1.00–1.05), and SBP (adjusted OR, 1.05; 95% CI, 1.02–1.07) to be positively associated with the presence of ECG-LVH. Obesity (adjusted OR, 0.58; 95% CI, 0.34–1.01) was not significantly associated with the presence of ECG-LVH (Table 2). After introducing monthly household income, regular exercise, a history of stroke, a history of dyslipidemia, FBS, uric acid or hemoglobin levels (Supplementary Table 2, models A, C–E, and G–I) into the model, these relations of male sex, age and SBP to the presence of ECGLVH remained unchanged in terms of statistical significance and direction. In addition, adjustments for monthly household income or a history of stroke showed a statistically significant inverse relationship with regard to obesity and the presence of ECG-LVH. The effects of monthly household income, regular exercise, a history of stroke, a history of dyslipidemia, FBS, uric acid, and hemoglobin levels were not statistically significant. Adjustment for smoking (Supplementary Table 2, model B) attenuated the relations of sex to the presence of ECGLVH. Adjustments for PR or AST (Supplementary Table 2, models F and J) attenuated the relations of age, while a significant inverse relation of obesity to the presence of ECG-LVH was observed. The effects of smoking, PR and AST were not statistically significant.

Multiple logistic regression which included the independent variables of sex, age, systolic blood pressure and obesity in clinically normotensive men and women (combined) without a history of hypertension according to the presence of electrocardiographic left ventricular hypertrophy or normal sinus rhythm (no. of subjects with information=2,582)
Analyzing the data for men and women separately, the associations of clinical variables were sustained virtually. In men, except for one model that adjusted for regular exercise, there was a significant increase in the risk of the presence of ECG-LVH with increase in SBP (Table 3; Supplementary Table 3, models A, B, and D–J). In women, all models except for one model that adjusted for menopause, showed a positive relation of age to the presence of ECG-LVH (Table 3; Supplementary Table 3, models A–J); and after adjustments for monthly household income or smoking, there was a significant increase in the risk of the presence of ECG-LVH with an increase in SBP (Supplementary Table 3, models A and B). However, a statistically significant positive relation of smoking and regular exercise; an inverse relation of PR to the presence of ECG-LVH were found only in men (Supplementary Table 3, models B, C, and F), and an inverse association with uric acid was found only in women (Supplementary Table 3, model H).
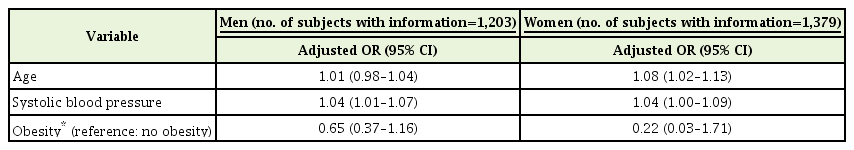
Multiple logistic regression which included the independent variables of age, systolic blood pressure and obesity in clinically normotensive women and men (separately) without a history of hypertension according to the presence of electrocardiographic left ventricular hypertrophy or normal sinus rhythm
3. Analyses Stratified according to Regular Exercise
Overall, 120 of 784 female participants (15.3%) practiced exercise regularly, but none showed evidence of ECG-LVH. In contrast, men who were <60 years of age without a prior history of stroke, with cardiac disease including ischemic heart disease, diabetes, and dyslipidemia as well as hypertension, nine of 106 participants (8.5%) in the regular exercise group and 16 of the 382 participants (4.2%) in the non-regular exercise group exhibited ECG-LVH. Therefore, we conducted regular exercise-stratified analyses only in men.
In univariable logistic regression analysis, the factors significantly associated with increased risk of ECG-LVH were: higher DBP in the non-regular exercise group and none of variables in the regular exercise group respectively (Supplementary Table 4). In addition to DBP, all of the significant variables in multiple logistic regression analysis for data in men and women (separately) and obesity were also included in multivariable logistic regression models.
Multivariable logistic regression analysis in the non-regular exercise group showed that age and SBP were positively associated with the presence of ECG-LVH, with an adjusted ORs of 1.08 (95% CI, 1.01–1.16) and 1.06 (95% CI, 1.01–1.12), respectively, while obesity was negatively associated (adjusted OR, 0.20; 95% CI, 0.04–0.93) (Table 4). After introducing smoking, PR, or uric acid (Supplementary Table 5, models A, C and D) into the model, these relations remained unchanged both in statistical significance and direction. The effects of smoking, PR, and uric acid were not statistically significant. Adjustment for DBP (Supplementary Table 5, model B) attenuated the relations of age and SBP to the presence of ECG-LVH, and the effect of DBP was not statistically significant. When DBP was added into the analysis instead of SBP (Supplementary Table 5, model E); the relations were similar, but age was no longer significantly associated with the presence of ECG-LVH, and the effect of DBP was statistically significant. On the other hand, in the regular exercise group, neither age nor SBP was significantly associated with the presence of ECG-LVH (Table 4). Only PR showed a statistically significant (inverse) relation to the presence of ECG-LVH in the regular-exercise group (Supplementary Table 5, model C).
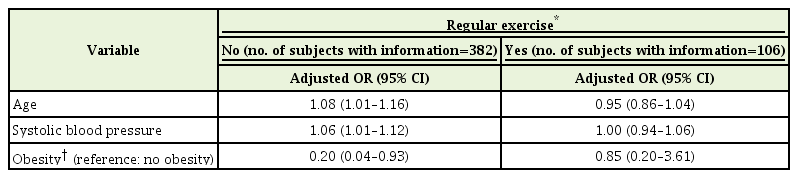
Regular exercise-stratified multiple logistic regression which included the independent variables of age, systolic blood pressure and obesity in clinically normotensive men without a history of hypertension according to the presence of electrocardiographic left ventricular hypertrophy or normal sinus rhythm
DISCUSSION
The presence of LVH is associated with a significantly increased risk of cardiovascular complications, irrespective of whether it is determined by ECG or ECHO [1,2]. In fact, ECHO is the gold standard for assessing anatomical LVH. While ECG shows a high specificity in diagnosing anatomical LVH, its sensitivity is relatively low [1]. However, considering that ECG-LVH may predict cardiovascular mortality and morbidity independently of ECHO-LVH and ECG provides information not available from ECHO (i.e., ECG-LVH may not depend solely on left ventricular mass suggesting that anatomical ECHO and electrical ECG versions might reflect different pathogeneses) [1], the interpretation of the clinical significance of ECG-LVH once discovered, rather than questioning whether or not to exclude the LVH diagnosis may differ in importance. The present study is, as far as we know, the first to investigate factors associated with ECG-LVH in both sexes by targeting only clinical normotensives with no prior history of hypertension from the Korean population. This information is clinically relevant as it may improve the usefulness of ECG because it facilitates clinical decisions associated with ECG-LVH.
For all participants, male sex, age, and SBP were independently and positively associated with the presence of ECG-LVH. Although it was found attenuated after adjustment for potential confounding factors, the association remained significant for most outcomes. These findings are consistent with those of previous studies. The prevalence of ECG-LVH increases with age [1,2,5]. In ECG-LVH according to Sokolow-Lyon criteria, there is a male predominance [1,5]. Furthermore, the incidence of ECG-LVH is closely associated with BP levels [1,2,5]. With respect to SBP, there are some possible mechanisms through which BP may influence left ventricular growth in our study of clinical normotensives with no prior history of hypertension. First, the association of SBP with an increased risk of ECG-LVH may be explained by responses to high normal BP. SBP levels within the normal limits have been shown to influence the increasing prevalence of LVH [16]. Second, it can be hypothesized that the association of ECG-LVH with male sex, older age, and relatively higher clinical SBP is a result of masked hypertension (MH) playing a significant role in the genesis of hypertrophy. MH is defined as normal BP on measurement in the clinic but a hypertensive BP level on ambulatory measurements [17]. The risk of cardiovascular morbidity and mortality in patients with MH is close to that of patients with uncontrolled hypertension [18]. A relatively higher SBP in the clinic is associated with MH in several studies [19-21], while other associated factors of ECG-LVH in our study, such as male sex and older age have also been shown to be factors associated with MH in previous studies [21]. Moreover, this explanation is in accordance with a previous report in which ECG-LVH was shown to be an independent determinant of MH [22].
In men, ex-smokers were associated with increased risk of ECG-LVH compared with never-smokers. Although the relationship was not statistically significant, current-smokers were also associated with increased risk of ECG-LVH. In agreement with our observation, hypertensive patients with ECG-LVH according to the Sokolow-Lyon criteria in other studies were more likely to be current smokers than participants without ECG-LVH [6]; and ever smokers in men [1]. Meanwhile, a significant effect of smoking on ECG-LVH was observed exclusively in men, but not in women. The fewer number of ex-smokers and currentsmokers in women compared to men might hinder an accurate estimation of the effects of smoking on ECG-LVH in women.
In women, uric acid was inversely associated with the presence of ECG-LVH. Unlike our results, previous studies have found that uric acid is independently and positively associated with ECG-LVH [6,23]. These contradictory results could be a result of confounders. Considering that the complex metabolic changes associated with menopause have been found to be linked to serum uric acid levels, and women taking HRT have been shown to have significantly lower serum uric acid concentration than those who did not take HRT [24]; we also assessed the relationship between uric acid levels and ECG-LVH after adjustments for menopause or HRT (Supplementary Table 6). Adjustment for HRT attenuated the association between uric acid and the presence of ECG-LVH (Supplementary Table 6, models B and D). However, information on HRT in the present study was available for only about one tenth of the female participants. The analysis with HRT as covariate was limited by the small sample size and was not adequately powered to definitely determine whether HRT may moderate the effects of uric acid on ECG-LVH. On the other hand, we identified an association between uric acid levels and ECG-LVH status in women, but not in men. The association of uric acid with menopause and HRT outlined above might also explain sex differences.
With respect to physiologic LVH which is a functional and structural adaptation of the heart to vigorous physical training and differs fundamentally from pathologic LVH [25], we further examined factors associated with ECG-LVH in regular exercise-stratified analyses setting a limit to age and diagnosis histories. Analyses were performed only in relatively healthy young men, because among women who exercised regularly, none exhibited ECG-LVH, which might be related to previous report that in comparison to men, athletic training in women athletes was not a stimulus for substantial increase in left ventricular wall thickness [26]. In the non-regular exercise group, older age and higher SBP were associated with an increased risk of ECG-LVH, while obesity was associated with a decreased risk of ECG-LVH. In the regular exercise group, only PR showed a statistically significant (inverse) association with the presence of ECG-LVH. Neither age nor SBP was significantly associated with the presence of ECG-LVH. In that specific cardiovascular risk factors such as higher BP and older age were not associated - in terms of direction, even showing inverse relations of age and SBP to the presence of ECG-LVH – ECG-LVH in the regular exercise group might be fundamentally different from ECG-LVH in the non-regular group in the context of physiological versus pathological LVH. The inverse relationship of PR with the presence of ECG-LVH in the regular exercise group is in accordance with other studies which revealed that resting sinus bradycardia was the most frequent ECG finding of well-conditioned athletes [27].
On the other hand, ECG-LVH according to the Cornell criteria has been found to be positively related to obesity, consistent with the known relationship of anatomic LVH to obesity [2,6,8]. In contrast, ECG-LVH according to the Sokolow–Lyon criteria has been found to be negatively associated with obesity in hypertensive patients as previously described in the literature. This suggests that the Sokolow–Lyon criteria identifies ECG-LVH in which obesity does not play a significant role in the genesis of hypertrophy and, in addition, reflects the negative influence of obesity on precordial voltage amplitudes in hypertensives and subsequent a lower sensitivity of the Sokolow-Lyon criteria for LVH in obese patients [1,6]. In this study, to increase the sensitivity for detecting ECG-LVH, both the Cornell and the Sokolow-Lyon criteria were accepted for defining ECG-LVH, because the Cornell criteria have been shown to be less dependent on body status also in the Korean population [11]. However, of the total 76 participants with ECG-LVH who were normotensives on clinical measurement and did not have a prior diagnosis of hypertension, only three participants fulfilled both the Sokolow–Lyon criteria and the Cornell criteria, and only one participant fulfilled the Cornell criteria alone. Consequently, the presence of ECG-LVH in our study was defined mostly by the Sokolow–Lyon criteria, and although not statistically significant except for the four models that adjusted for monthly household income, a history of stroke, PR, or AST in all participants, the inverse effects of obesity in our study were in accordance with the results of other studies on hypertensive patients [1,6]. Regarding statistical significance, men with ECG-LVH were more likely to do regular exercise than those with NSR (Table 1), and this might attenuate the association of obesity with the presence of ECG-LVH. Because the relationship between obesity and ECG-LVH in past investigations was observed in hypertensive patients [1,6], the ECG-LVH in hypertensive patients was a manifestation of heart disease, and physiologic LVH is different from pathologic LVH in nature [25]; including men who did regular exercise – who were more likely to have physiologic LVH rather than pathologic LVH compared with those who did not regular exercise - in analyses might attenuate the effects of obesity on ECG-LVH. Further regular exercise-stratified multivariable logistic regression analyses suggested that in the non-regular exercise group, obesity was independently and negatively associated with the presence of ECG-LVH, while in the regular exercise group, obesity showed no statistical significance, possibly providing an explanation for the non-significant association found on multivariable analyses in all participants. Meanwhile, in the non-regular exercise group, the presence of ECG-LVH was also defined mostly by the Sokolow-Lyon criteria (n for total ECG-LVH=16; n for fulfilling the Sokolow–Lyon criteria alone=15; n for fulfilling both the Sokolow–Lyon criteria and the Cornell criteria=1; n for fulfilling the Cornell criteria alone=0). As indicated above, it must be admitted that the inverse relationship of obesity with the presence of ECG-LVH in the non-regular exercise group may be an artifact of the ECG-LVH measurement by the Sokolow-Lyon criteria, since obesity of the chest wall will have a negative influence on precordial voltage amplitudes. Moreover, previous investigators have found a positive association of obesity with ECHO-LVH [2,6,8] or the ECG-LVH defined by the Cornell criteria [1,6], as described above.
Our study has some limitations. Owing to the relatively small number of case participants, it is possible that the statistical power for assessment was insufficient especially in analyzing the data in men and women separately.
Indeed, analysing the data in men and women separately, the statistical significance of relation of age to the presence of ECG-LVH was observed only in women, and the statistical significance of relation of SBP to the presence of ECG-LVH was observed only in men besides two models of female analysis. However, when we analyzed participants who did not exercise regularly in the regular exercise-stratified analyses [28], the relations of age and SBP to the presence of ECG-LVH remained virtually the same for all participants, as noted. It is possible that the sex difference observed when we analyzed the data in men and women separately, could be explained by sex-specific, relatively close or weak relationships between ECG-LVH and other cardiovascular risk factors [3].
The study was cross-sectional in design; therefore, we could not identify the causal relationships between ECG-LVH and several factors. In this context, ECG-LVH in the regular exercise group might not have been a consequence of exercise, nor representative of physiologic LVH, although our observations showed significant differences between the regular exercise group and the non-regular exercise group. Further longitudinal studies will be needed in the future.
In addition, we had intended to apply the two most widely used ECG-LVH criteria—the Sokolow-Lyon and the Cornell criteria. In recent guidelines, detection of ECG-LVH defined according to the Sokolow-Lyon and Cornell criteria appears in the list of routine tests to determine target organ damage in hypertension [9]. However, the presence of ECG-LVH in our study was defined mostly by the Sokolow–Lyon criteria as mentioned above, and thus, we were unable to determine whether combining the two ECG-LVH criteria would produce the same results. Furthermore, eligible case participants included those with only ECG-LVH defined according to the Sokolow-Lyon and Cornell criteria without other ECG findings including ST-T changes and arrhythmias, and although this restriction could reduce any potential confounding factors, participants selected on the basis of isolated voltage ECG-LVH might not be common patients with ECG-LVH. Thus, caution must be exercised when our results are extrapolated and applied to the general population and other specific subgroups.
In conclusion, male sex, older age, and higher SBP were associated with ECG-LVH in participants who were normotensives on clinical measurement with no prior diagnosis of hypertension. The positive relation of smoking and regular exercise; inverse relation of PR to the presence of ECG-LVH were found only in men. The inverse relation of uric acid level was found only in women. Detailed analyses of relatively healthy young men according to whether or not they exercise regularly showed that in the non-regular exercise group, age and SBP to be positively related to the presence of ECG-LVH, while obesity to be negatively related. However, in the regular exercise group, neither age nor SBP was significantly associated with the presence of ECG-LVH, and only PR showed a statistically significant (inverse) association. The varying risk factor profiles associated with ECG-LVH based on sex and whether or not to exercise in our study may help to elucidate the ECG-LVH in clinical normotensives with no prior history of hypertension. Further longitudinal studies in general populations will be needed to better understand the development of ECG-LVH.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Supplementary Tables 1–6 can be found via https://doi.org/10.4082/kjfm.18.0008.