Analysis of the Time Interval between the Physician Order for Life-Sustaining Treatment Completion and Death
Article information
Abstract
Background
This study aimed to explore the time interval distribution pattern between the Physicians Order for Life-Sustaining Treatment (POLST) form completion and death at a tertiary hospital in South Korea. It also examined the association between various independent parameters and POLST form completion timing.
Methods
A total of 150 critically ill patients admitted to Korea University Guro Hospital between June 1, 2018 and December 31, 2018 who completed the POLST form were retrospectively analyzed and included in this study. Data were analyzed with descriptive statistics, and group comparisons were performed using the chi-square test for categorical variables. Fisher’s exact test was also used to compare cancer versus non-cancer groups.
Results
More than half the decedents (54.7%) completed their POLST within 15 days of death and 73.4% within 30 days. The non-cancer group had the highest percentage of patients (77.8%) who died within 15 days of POLST form completion while the colorectal (39.1%) and other cancer (37.5%) groups had the lowest (P=0.336).
Conclusion
Our findings demonstrated a current need for more explicit guidance to assist physicians with initiating more timely, proactive end-of-life discussions.
INTRODUCTION
The number of elderly residents is rapidly increasing in South Korea. In recent years, there has been an increasing number of cases where critically ill, end-of-life patients were acutely treated against their wishes to face a natural death. Previous studies have shown that acute, aggressive end-of-life medical treatments are associated with a decreased patient quality of life as well as subsequent bereaved family member regret [1]. Consequently, a national consensus has emerged that critically ill patients should be allowed to make decisions about their treatment plans based on their values and goals. In response to the changing public attitude mentioned above, the Act on Decisions on Life-Sustaining Treatment for Patients in Hospice and Palliative Care or at the End of Life (hereafter known as the law) was first introduced in February 2016. After a 2-year trial run, the law went into full effect on February 4, 2018.
The core component of the law is the Physicians Order for Life-Sustaining Treatment (POLST) which aims to improve end-of-life care by honoring the values and goals of critically ill patients. The POLST allows these patients to waive unwanted medical interventions such as cardiopulmonary resuscitation, mechanical ventilation, hemodialysis, and anticancer drug administration when there is no chance the disease progression will be reversed. In March 2019, an amendment went into effect that increased the types of waivable medical treatments to include extracorporeal life support for the heart or lungs, blood transfusions, and vasopressor use. As a result of this amendment, the POLST is now a stronger tool giving critically ill patients the right to choose a natural death.
End-of-life care offers critically ill patients high-quality care attuned to their values [2,3]. In other words, end-of-life care is substantially different from acute treatment as it encompasses not only a reduction in acute medical interventions but also emotional and spiritual care. Given that end-of-life care can only start after the POLST form is completed, timely completion of the form is critical to ensure that patients are given sufficient time to experience end-of-life based on their goals and values. Previous studies demonstrated that POLST form completion leads to decreased incidents of patients receiving unwanted medical interventions [4,5]. Therefore, in order for the law to be successfully implemented in the future and to offer physicians additional guidance, further discussions concerning appropriate POLST form completion timing are needed. Defining appropriate POLST form completion timing ensures that critically ill patients can receive high-quality care that provides them with an opportunity to experience the last phase of life with dignity, and also eases bereaved family member’s pain after the patient’s death. It has been more than a year since the law first went into effect, and it is now worth examining POLST form completion timing to gauge the impact of the law in current practice.
The purpose of this study was to examine the time interval distribution pattern between POLST form completion and death at a tertiary hospital in South Korea. The study also analyzed the association between various independent parameters and POLST form completion. Overall, this study intended to provide valuable insight into improving the implementation of the law by exploring POLST form completion timing.
METHODS
1. Sample and Data Source
The subjects were patients with a terminal illness admitted to Korea University Guro Hospital between June 1, 2018 and December 31, 2018 who completed the POLST form. The medical records of 203 patients were reviewed. Of these patients, 53 were still surviving and excluded because this study analyzed the time interval between POLST completion and death. A total of 150 patients were retrospectively analyzed and included in this study. The primary outcome was the time interval difference according to primary clinical diagnosis and age.
Demographic data including age, sex, primary diagnosis, admission date, POLST form completion date, and death date were collected from the hospital POLST task force with their permission. The primary clinical diagnosis was further categorized into solid tumor, hematologic malignancy, and organ failure. This retrospective study was approved by the Institutional Review Board of Korea University Guro Hospital (IRB approval no., 2019GR0086) and the duty of informed consent was exempted by the board due to the retrospective nature of this study involving the deceased.
2. Statistical Analysis
First, descriptive statistics and frequency distributions for different variables were examined. Group comparisons were performed using chi-square for categorical variables to determine statistically significant relationships between time interval and various independent parameters. Fisher’s exact test was also used to compare cancer versus non-cancer groups with two sets of time intervals categorized by a period of 15 days. Differences with a P-value of 0.05 and below were considered statistically significant. Analyses were conducted using IBM SPSS ver. 25.0 (IBM Corp, Armonk, NY, USA).
RESULTS
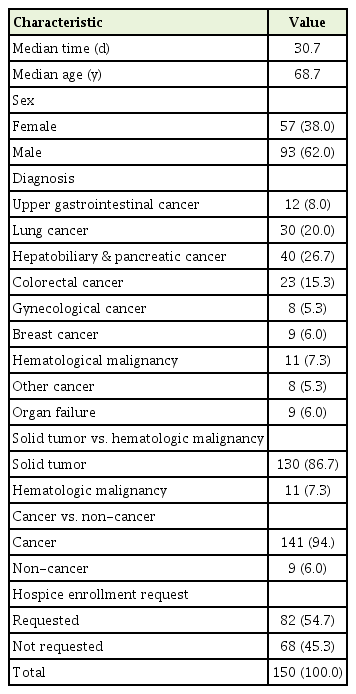
The sample characteristics are summarized in Table 1. The decedents had a mean age of 68.7 years at the time of POLST form completion. The median time between POLST form completion and death was 30.7 days. Of the patients included, 62% were male, 94% had cancer, and 54.7% had requested hospice admission. Hepatobiliary and pancreatic cancer was the most common (26.7%) diagnosis in the groups followed by lung (20%), colorectal (15.3%), and upper gastrointestinal cancer (8%). Non-cancer patient diagnoses constituted 6% of the decedents and included organ failure from chronic heart, lung, liver, and kidney disease. Of the different cancer group types, 86% of the patients had solid tumors.
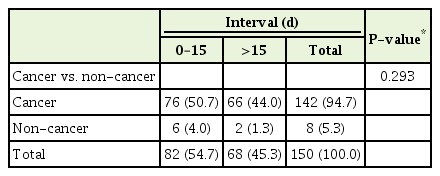
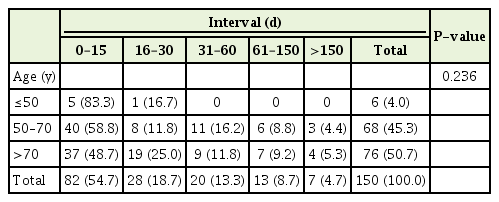
Table 2 illustrates the primary clinical diagnosis related to the time interval. The non-cancer group had the highest percentage of decedents (77.8%) who died within 15 days of POLST completion while the colorectal (39.1%) and other cancer (37.5%) groups had the lowest. However, the differences in time interval by diagnosis were statistically insignificant (P=0.336). Using the chi-square test for the comparison between cancer and non-cancer groups, the non-cancer group had a higher percentage of decedents who died within 15 days of form completion than the cancer group; however, the results were also not statistically significant (P=0.241). Given the small size of the other cancer group, we also used Fisher’s exact test to compare the groups with the 15-day time interval (Table 3). Again, the results were not statistically significant (P=0.293). When the primary clinical diagnosis groups were compared, 53.8% of the solid tumor group died within 15 days of POLST form completion compared to 45.5% of the hematological malignancy group (P=0.887). Table 4 outlines the time intervals among three different age groups. The group including those 50 years of age or younger represented the highest percentage of patients (83.3%) who completed the POLST form within 15 days of dying whereas the group 70 years of age or older had the lowest at 48.7% (P=0.236).
Table 5 depicts the time interval distribution pattern in days for all patients. Of these patients, 54.7% completed the POLST form within 15 days of dying and 73.4% died within 30 days of form completion.
DISCUSSION
This study intended to examine the association between various independent parameters and the time interval from POLST form completion to death. The independent parameters used in this study, including age, diagnosis, cancer versus non-cancer, and solid tumor versus hematological malignancy, were not statistically significant concerning the time interval. We hypothesize that this may be due to the small sample size. Compared to a cohort study involving Oregon POLST Registry decedents in the United States, Zive et al. [6] concluded that the cause of death influenced the time interval where the cancer group completed the form closest to death. As more POLST form data accumulates at tertiary hospitals in South Korea, future research is needed to re-address the relationship between cause of death and POLST completion timing.
Regarding the time interval distribution pattern, the fact that more than half the decedents (54.7%) completed their POLST within 15 days of death and 73.4% within 30 days demonstrates the current practice of POLST form completion being delayed until the final days of life (Table 5). Compared to the results of a cross-sectional study conducted in New York state in the United States, Araw et al. [7] showed that 22% of patients died within 30 days of form completion. With regard to the median time interval between POLST form completion and death, which were 49 days in a study of Araw et al. [7] and 40 days in a study of Zive et al., [6] were also longer than 30.7 days in our study (Table 1).
The main goal of the law is to enable end-of-life care to be a planned part of a terminal patient’s life. Therefore, it should be emphasized that the effectiveness of the law largely depends on ideal timing of POLST form completion. Arguably, when health professionals treat patients in ways that do not match patient values and wishes, grave medical errors may occur [3,8]. Previous studies identified that acute medical treatments administered against patient wishes may result in higher medical costs and decreased quality of life [9-11]. In this sense, the study findings demonstrate an urgent need for clearer, more detailed guidance to assist healthcare professionals with deciding ideal timing for POLST form completion.
Optimal timing for POLST for completion has been an important topic for discussion in the United States as well. If completed too early, the POLST form might lead to treatment option reduction [12] before the patient decides on their preferences [13]. On the other hand, if the POLST form is completed close to death, the delay can significantly decrease the law’s effectiveness. Moreover, timely POLST form completion can provide patients with an option to be referred to hospice care at an appropriate time and a more timely hospice admission can offer patients the many benefits of end-of-life care [14].
There are various reasons for POLST form completion delays. Many physicians tend not to discuss end-of-life care options with critically ill patients until a crisis arises [15,16]. Prognostic uncertainty is another reason physicians tend to delay initiating end-of-life discussions [16], reducing the chance for patients to receive high-quality care that meets their personal needs [17]. Other clinical demands often take place before physicians decide to initiate end-of-life discussions [18].
In an effort to prevent POLST form completion delays, a growing consensus that clinicians should proactively initiate a “goals of care” conversation with critically ill patients has emerged among physicians and patients alike in the United States [19]. Similarly, a national cross-sectional study in Japan concluded that end-of-life discussions about a preferred treatment plan should be initiated early with advanced cancer patients [17]. High-quality communication between clinicians and patients or their surrogates is the key to achieving timely end-of-life care [3].
The interpersonal process of shared decision making between health professionals and patients can be achieved only through good communication [20], which ultimately enhances trust between them [21]. Shared decision making can also provide patient family members with emotional support as well as add to their overall satisfaction with medical care [22]. In a cohort study involving a group of ovarian cancer patients, the patients who had earlier end-of-life discussions received higher quality end-of-life care [23]. Therefore, systematic guidance for initiating earlier end-of-life discussions is necessary to improve the current situation of POLST form completion delays.
The U.S. Veterans Health Administration (VHA) has more than 9 million veterans enrolled and is an example of a systematic approach. Its program offers medical professionals the necessary training to proactively identify high-risk patients and document the patients’ goals regarding their medical treatment options [24]. For instance, the VHA trains teams to use comprehensive analytical tools such as the Care Assessment Needs score for screening high-risk patients and assist medical professionals with proactively initiating end-of-life discussions [24].
There are several limitations to our study. First, the lack of associations between different parameters and time interval may be due to the small sample size. Second, concerning the classification method, patients who were previously diagnosed with cancer at any point in their lives were classified as cancer patients regardless of the actual cause of death. Therefore, the possibility exists that our classification method may have altered the statistical results. For instance, a patient who had a history of gastrectomy due to stomach cancer and died from acute complications related to heart failure may have fit better in the organ failure group than the cancer group. Further research with a larger sample size is needed to examine the relationships between different parameters and POLST timing to shed a more accurate light on the factors that could influence the timing of end-of-life discussions. Nevertheless, to our knowledge, this is the first study to explore POLST form completion timing in South Korea to date and it is worthwhile to note that our study emphasized the importance of timely communication for shared decision making and, hence, the necessity for physician guidance on proactively initiating end-of-life discussions.
In conclusion, this study aimed to explore the time interval distribution pattern from POLST form completion to death. It also examined associations between various independent parameters and time intervals. The findings of our study indicated that POLST form completion is often delayed until the last days of life. However, there were no statistically significant relationships between selected parameters and time intervals. In conclusion, the results of our study demonstrated a current need for clearer guidance to assist physicians with initiating endlife-care discussions in a more timely, proactive manner.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.