Association between Sleep Duration and Presbycusis in Korean Adults: Korea National Health and Nutrition Examination Survey
Article information
Abstract
Background
Sleep duration is associated with hearing loss, especially presbycusis, which is the most common type of hearing loss; however, there is limited evidence regarding this association among the Korean population. We aimed to determine the relationship between sleep duration and high-frequency hearing loss in Korean adults aged ≥40 years.
Methods
We examined 5,547 Korean adults aged ≥40 years who completed audiometric tests and questionnaires regarding sleep duration during the 2010–2012 cycle of the Korea National Health and Nutrition Examination Survey. Mild presbycusis was defined as >25 decibels (dB) and <40 dB, whereas moderate-to-severe presbycusis was defined as >40 dB pure tone averages at high frequencies (3,000, 4,000, and 6,000 Hz) for both ears. Additionally, the sleep duration was divided into quartiles. Odds ratios and 95% confidence intervals were estimated using multivariable logistic regression after adjusting for covariates.
Results
The prevalence of presbycusis in South Korean adults was 62.1%, of which 61.4% showed moderate to severe presbycusis. The incidence of moderate-to-severe, but not mild, presbycusis showed a significant positive correlation with sleep duration.
Conclusion
Our findings suggest that sleep duration is associated with the prevalence of presbycusis.
INTRODUCTION
Hearing loss is among the most prevalent chronic diseases following arthritis and hypertension, with presbycusis being the most common type of hearing loss [1]. Presbycusis is defined as hearing loss attributable to age-related cochlear degeneration with bilateral, symmetrical, and gradual progression. Hearing loss interferes with the ability to understand language sounds, causing difficulties in communication and learning, thereby reducing productivity and social isolation. Furthermore, especially among older adults, hearing loss can contribute to difficulties in communicative relationships, reduced social and emotional interactions, and even depression. Approximately 33.3% and 9% of individuals with and without hearing loss are in fair and poor health, respectively [1-3].
In South Korea, the overall prevalence of high-frequency hearing loss is 36.85%, and bilateral hearing loss is positively correlated with age [3]. Since hearing loss is expected to increase with population aging, the risk factors for presbycusis should be determined. In Korea, hearing loss is associated with various factors including lifestyle parameters, such as smoking, high-risk alcohol use, education level, and employment status, and comorbidities, such as hypertension, dyslipidemia, stroke, obesity, arthritis, diabetes, depression, renal failure, age, and sex [4].
Sleep plays an important physiological role in health [5]. Sleep duration is strongly related to several health problems, including metabolic [6], cardiovascular [7], and psychological disorders [8]. Sleep duration and the incidence of hearing loss (especially at high frequencies) are positively correlated [9]. Furthermore, sleep deprivation in animal models induces hearing loss [10].
Although the pathogenesis of presbycusis remains unclear, several studies have investigated the mechanisms that underlie senile hearing loss. Presbycusis is characterized by overall loss of hair cells, decreased number of auditory nerve cells, hyalinization and stiffening of the cochlear basement membrane, and restricted hair cell metabolism due to stria vascularis degeneration. Some patients may present with two or more of these features, while others may present with none [1]. Sleep duration is associated with auditory radiation microstructure; hence, disturbance in the auditory microstructure may result in hearing loss [11]; however, there is limited clinical evidence regarding this effect among Koreans.
We examined the relationship between sleep duration and high-frequency hearing loss in Korean adults aged ≥40 years.
METHODS
1. Study Population
We examined 5,547 Korean adults aged ≥40 years who completed audiometric tests and questionnaires regarding sleep duration in the 2010–2012 cycle of the Korea National Health and Nutrition Examination Survey (KNHANES).
2. Data Collection
Participants underwent a standardized interview regarding their demographic, socioeconomic, and clinical characteristics, including sex, age, educational status, employment status, and various health-related information. Clinical characteristics included medication use, family history, smoking habit, alcohol use, previous and current diseases, and noise exposure.
Educational status was categorized as elementary school graduate or below, middle school graduate, high school graduate, or above university. Occupation status was classified as either employed or unemployed. Smoking habits were classified as a non-smoker, ex-smoker, or current smoker. High-risk alcohol use was defined as drinking seven and five cups for men and women, respectively, as well as drinking more than the average intake at a time and twice or more a week. High-risk alcohol use was categorized as none, once a month or less, once a week, or nearly daily. Chronic noise exposure was defined as exposure to the noise inside or outside the workplace or using earphones in loud places; acute noise exposure was defined as exposure to transient noise [12].
The clinical characteristics included waist circumference, hypertension, dyslipidemia, arthritis, diabetes, depression, obesity, stroke, and renal failure. Waist circumference was measured using standardized equipment and techniques. Abdominal obesity was defined as waist circumference ≥90 cm and ≥85 cm for men and women, respectively. Blood pressure was measured using a sphygmomanometer with the patient seated. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medications. Diabetes was defined as fasting plasma glucose level ≥126 mg/dL, use of antidiabetics or insulin injections, or previous diagnosis by a physician. Dyslipidemia was defined as lowdensity lipoprotein cholesterol levels ≥130 mg/dL, triglycerides ≥150 mg/dL, high-density lipoprotein cholesterol ≤40 mg/dL for men and ≤50 mg/dL for women, or the use of serum lipid-lowering medications. Obesity was defined as a body mass index ≥25 kg/m2. Participants were asked if they had been diagnosed with or were taking medication for osteoarthritis, depression, stroke, and renal failure.
3. Audiometric Measure
Pure-tone audiometry was performed on participants aged ≥12 years using an automatic audiometer (SA-203; Entomed, Malmö, Sweden). A trained otorhinolaryngologist performed the examination in a double-walled soundproof booth. The hearing thresholds were obtained for each ear at six frequencies (0.5, 1, 2, 3, 4, and 6 kHz). The Korean Society of Otorhinolaryngology-Head and Neck Surgery provided survey instructions, implemented on-site quality control measures, and examined the reliability of the results. Mild presbycusis was defined as pure tone averages >25 decibels (dB) and ≤40 dB at high frequencies (3, 4, and 6 kHz) bilaterally, whereas moderate-to-severe presbycusis was defined as >40 dB.
4. Sleep Measurement
Participants aged ≥40 years completed a questionnaire regarding sleep, where they provided their average daily sleep duration. Sleep duration was divided into quartiles as follows: ≤6 hours (Q1), 7 hours (Q2), 8 hours (Q3), and >8 hours (Q4). Subsequently, we examined the relationship between sleep duration and the prevalence of presbycusis.
5. Statistical Analyses
We obtained odds ratios and 95% confidence intervals with adjustment for education level, employment status, smoking, high-risk alcohol use, previous noise exposure, and comorbidities of hypertension, dyslipidemia, arthritis, diabetes, depression, and renal failure.
The chi-square test was used to examine the distribution of demographic, socioeconomic, and clinical characteristics. Multivariable-adjusted logistic regression was used to examine the association between sleep duration and the prevalence of presbycusis.
6. Ethics Statement
The study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB approval no., 05-2022-168). The requirement for informed consent was waived because the participants’ consent was obtained from the KNHANES. The dataset is stored in a public domain and does not include individually identifiable information.
RESULTS
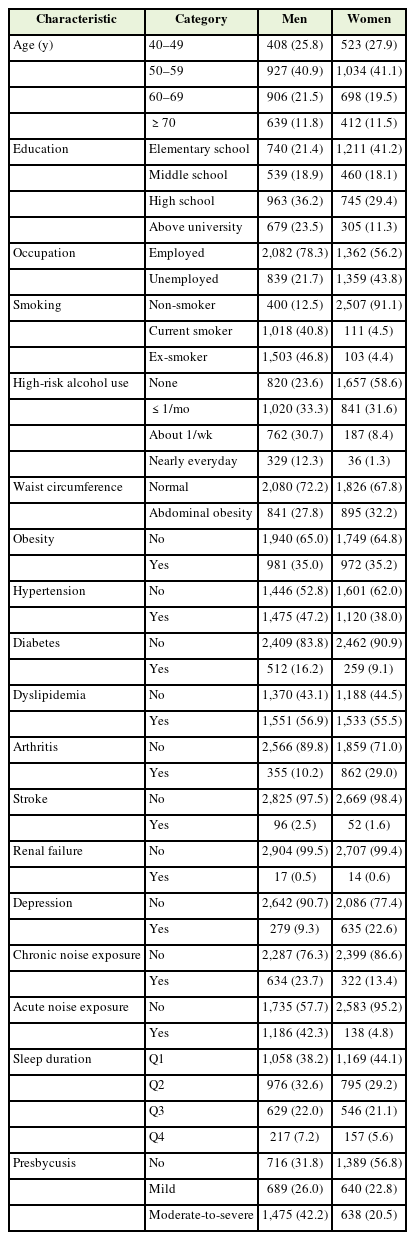
Among the 25,534 participants who completed the audiometric tests and questionnaires regarding sleep duration, we included those aged ≥40 years and excluded those with missing values of interest and potential confounders. Finally, 5,547 participants were included in this study. There were 2,880 men (57.2%) and 2,667 women (42.8%) (Table 1). The prevalence of hypertension and diabetes mellitus was 46.8% and 13.9%, respectively. The prevalence rates of chronic and acute noise exposure were 17.2% and 23.9%, respectively. Moreover, 20.4% of the participants were current smokers. There were 3,442 patients (62.1%) with presbycusis, with 61.4% showing moderate-to-severe presbycusis.
Men were more likely to be diagnosed with presbycusis than women; moreover, the prevalence of hearing loss increased with age (Table 2). Participants with a low educational level and an unemployed state were more likely to be diagnosed with presbycusis. Among clinical characteristics, hypertension, diabetes, arthritis, stroke, and renal failure were associated with the prevalence of presbycusis. Previous noise exposure was correlated with hearing loss.
Table 3 shows the multivariable-adjusted associations between sleep duration and the severity of presbycusis. Moderate-to-severe, but not mild, presbycusis was significantly associated with sleep duration (P<0.05). Compared with patients with a sleep duration of ≤6 hours, those with a sleep duration of 7, 8, and >8 hours had a higher incidence of presbycusis by 24%, 27%, and 47%, respectively.
DISCUSSION
In this study of Korean individuals aged ≥40 years, sleep duration was significantly associated with moderate-to-mild, but not mild, presbycusis, which is inconsistent with a previous report. Specifically, this population-based cross-sectional study examined the relationship between sleep duration and hearing loss in Japanese individuals aged 20–79 years. Compared with sleep duration ≤5 hours, longer sleep duration categories were associated with hearing loss at 4 kHz, and a sleep duration ≥9 hours was associated with hearing loss at 1 kHz [13]. However, our study used pure-tone audiometry rather than single frequencies, which is more clinically relevant, and we included a nationally representative cohort of Korean adults aged ≥40 years.
The mechanisms underlying the association between sleep duration and hearing loss remain unclear. A previous study reported a negative relationship between mean sleep duration and fractional anisotropy, which is a widely used index for microstructural white matter integrity in auditory radiation [11]. However, the association of auditory radiation, which is the final part of the auditory pathway from the cochlea to the cerebral cortex, with sleep-hearing interaction remains unclear. Contrastingly, we observed a positive correlation between the incidence of moderate to severe presbycusis and sleep duration.
Long sleep duration is correlated with risk factors for cardiovascular factors; moreover, increased cardiovascular risk may contribute to hearing loss [7,9,14]. Disrupted blood flow and energy metabolism can cause loss of outer hair cells, resulting in hearing loss. In our study, stroke, a cardiovascular risk factor, was associated with presbycusis. Other factors related to presbycusis include sex, education level, and history of arthritis. Furthermore, otorhinolaryngology is not a major risk factor for senile hearing loss. Therefore, we concluded that the effect of otolaryngological diseases in this study did not differ from that of other chronic diseases [15,16].
Men are reportedly more likely to experience presbycusis than women [4,17]. This could be attributed to their relatively greater exposure to occupational noise and military experience in Korea. Additionally, because estrogen is necessary for normal hearing and hearing maintenance, it has a protective effect against presbycusis. Furthermore, differences in prenatal molding in auditory physiology may explain sexdependent high-frequency hearing loss [18].
This study had several limitations. First, it was a cross-sectional study; therefore, we could not determine a causal relationship. Second, data on several variables, including smoking history, noise exposure, and clinical history, were collected using self-report questionnaires. Therefore, there is a possibility of reporting or recall biases. Third, we could not consider the patients’ history of otologic disease or use of ototoxic drugs because of missing information. However, a study has investigated the positive correlation between sleep duration and prevalence of presbycusis, excluding participants with a history of otologic diseases [13]. Fourth, we did not consider other sleep characteristics, including snoring, stopping breathing, or excessive daytime sleepiness, which might be related to hearing loss. Future studies should consider these factors.
In conclusion, our findings showed that sleep duration positively correlated with presbycusis prevalence. Furthermore, sex, education level, and history of arthritis were related to hearing loss. Further studies are needed to determine the causal relationship between sleep duration and high-frequency hearing loss. Future prospective studies should also consider other sleep characteristics that might be related to hearing loss.
Notes
CORRECTION
This article was corrected on November 29, 2023 for an author affiliation.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors would like to thank the staff and participants of the Korea National Health and Nutrition Examination Survey.