 |
 |
- Search
| Korean J Fam Med > Volume 43(1); 2022 > Article |
|
Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
Figure.┬Ā1.
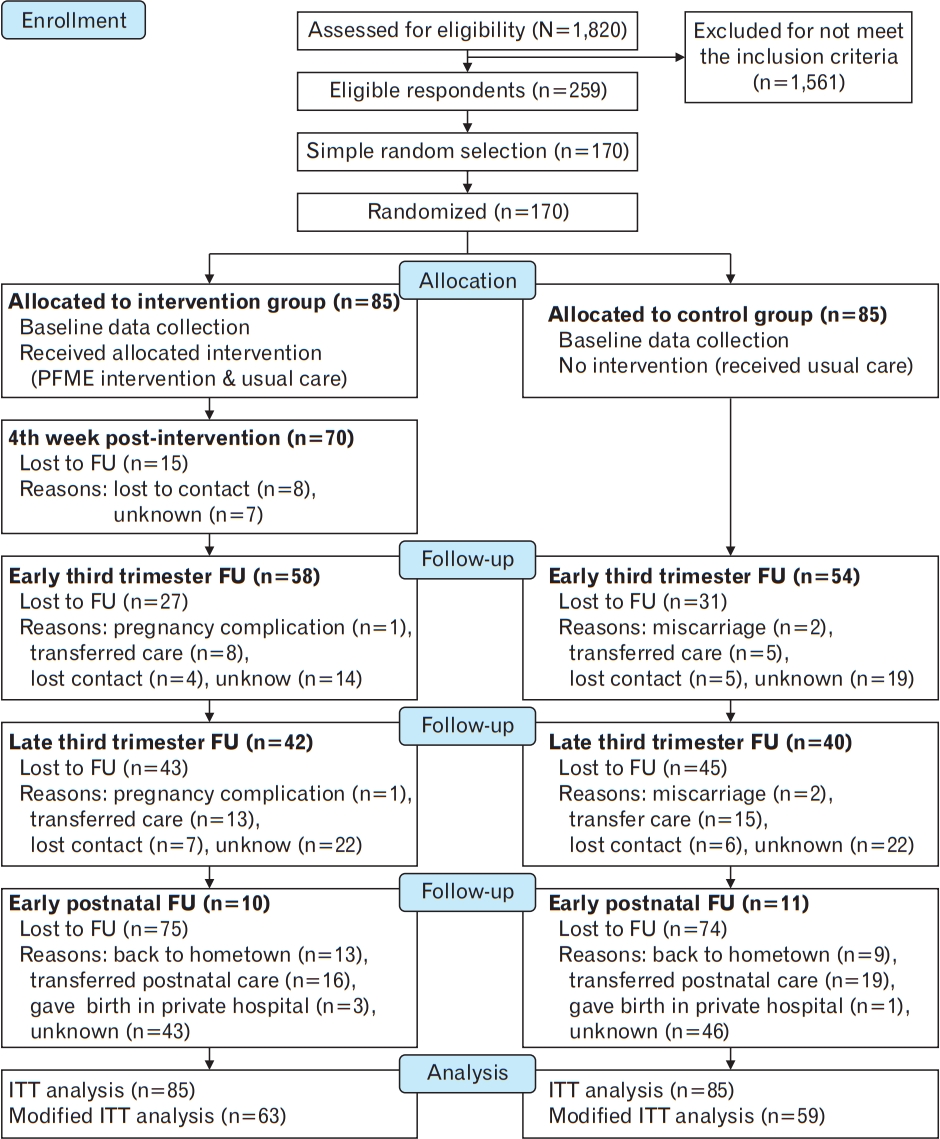
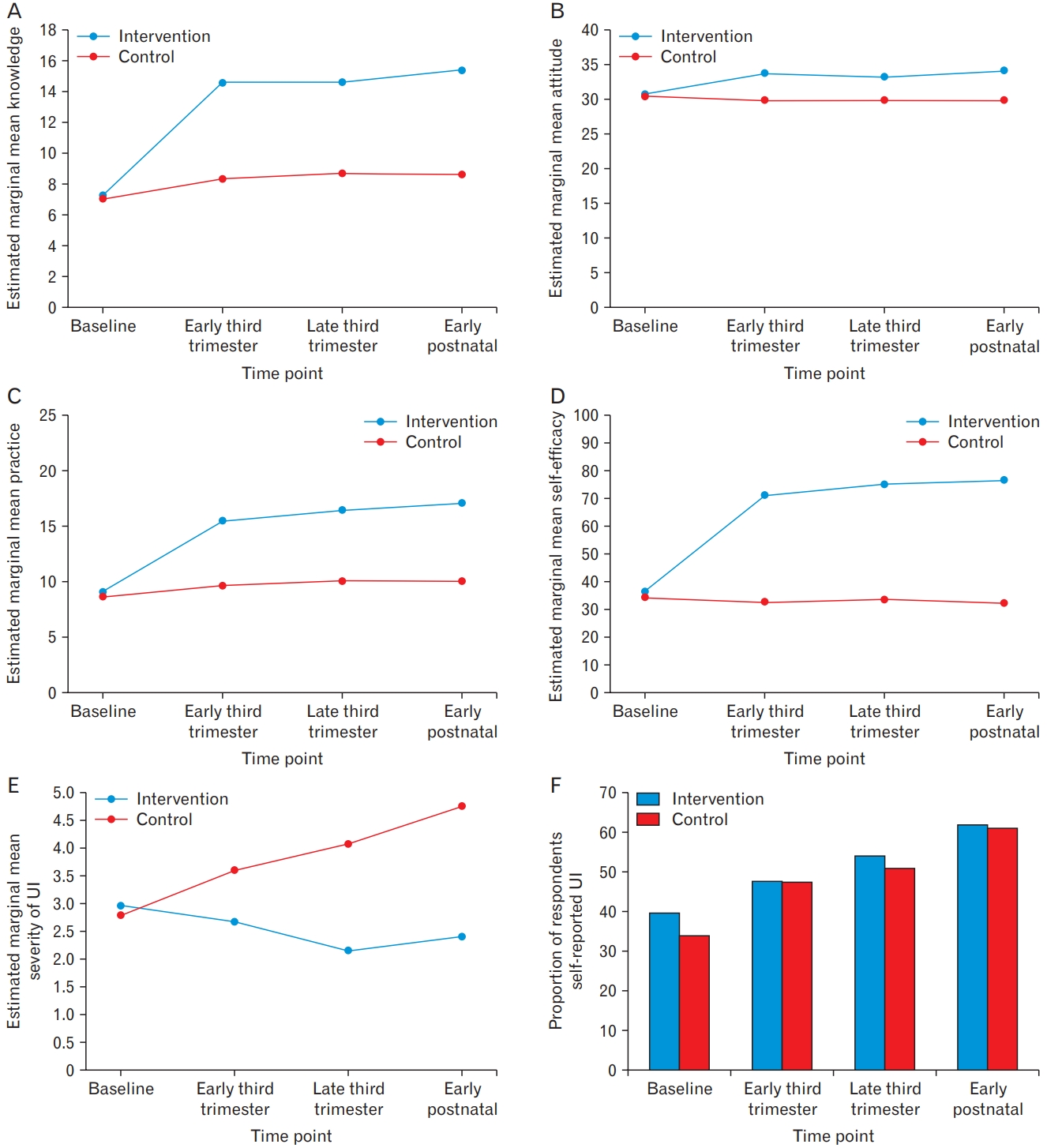
Figure.┬Ā2.
Table┬Ā1.
Table┬Ā2.
| Characteristic |
Baseline comparison |
Baseline comparison among completer (n=122) (included in primary analysis) |
Baseline comparison among completer and non-completer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group (n=85) | Control group (n=85) | P-value | Intervention group (n=63) | Control group (n=59) | P-value | Non-completer (n=48) | P-value | ||
| Age (y)* | 30.3┬▒5.1 | 30.9┬▒5.3 | 0.429 | 31.0┬▒4.6 | 31.3┬▒5.5 | 0.782 | 29.4┬▒5.5 | 0.046 | |
| Body mass index (kg/m2)* | 25.7┬▒5.6 | 27.5┬▒6.2 | 0.049 | 26.2┬▒5.5 | 27.4┬▒6.4 | 0.256 | 26.1┬▒5.9 | 0.482 | |
| Gestational (wk)* | 18.8┬▒2.2 | 18.8┬▒2.0 | 0.912 | 18.7┬▒2.2 | 18.9┬▒1.8 | 0.434 | 18.7┬▒2.3 | 0.870 | |
| Salary (RM)* | 2,760.0┬▒1,415.9 | 2,804.9┬▒1,407.5 | 0.864 | 2,820.6┬▒1,342.8 | 2,793.2┬▒1,412.8 | 0.926 | 2,695.4┬▒1,542.1 | 0.704 | |
| EthnicityŌĆĀ | 0.133 | 0.448 | 1.00 | ||||||
| ŌĆā | Malay | 75 (88.2) | 67 (78.8) | 55 (87.3) | 46 (78.0) | 41 (85.4) | |||
| Chinese | 3 (3.5) | 5 (5.9) | 2 (3.2) | 4 (6.8) | 2 (4.2) | ||||
| Indian | 4 (4.7) | 12 (14.1) | 4 (6.3) | 8 (13.6) | 4 (8.3) | ||||
| Others | 3 (3.5) | 1 (1.2) | 2 (3.2) | 1 (1.7) | 1 (2.1) | ||||
| EducationŌĆĪ | 0.093 | 0.025 | 0.463 | ||||||
| Primary | 1 (1.2) | 2 (2.4) | 0 | 2 (1.6) | 1 (2.1) | ||||
| Secondary | 26 (30.6) | 38 (44.7) | 17 (27.0) | 26 (44.1) | 21 (43.8) | ||||
| College/university | 58 (68.2) | 45 (52.9) | 46 (73.0) | 31 (52.5) | 26 (54.2) | ||||
| OccupationalŌĆĀ | 0.251 | 0.131 | 0.222 | ||||||
| Public sector employed | 31 (36.5) | 24 (28.2) | 27 (42.9) | 17 (28.8) | 11 (22.9) | ||||
| Private sector employed | 24 (28.2) | 24 (28.2) | 17 (27.0) | 19 (32.2) | 12 (25.0) | ||||
| Self-employed | 5 (5.9) | 3 (3.5) | 4 (6.3) | 1 (1.7) | 3 (6.3) | ||||
| Housewife | 23 (27.1) | 34 (40.0) | 14 (22.2) | 22 (37.3) | 21 (43.8) | ||||
| Others | 2 (2.4) | - | 1 (1.6) | 0 | 1 (0.6) | ||||
| ParityŌĆĪ | 0.752 | 0.701 | 0.022 | ||||||
| Nulliparous | 33 (38.8) | 31 (36.5) | 19 (30.2) | 20 (33.9) | 25 (52.1) | ||||
| Multiparous Ōēź1 | 52 (61.2) | 54 (63.5) | 44 (69.8) | 39 (66.1) | 23 (47.9) | ||||
| History of deliveryŌĆĪ | 0.612 | 0.938 | 0.065 | ||||||
| SVD | 35 (41.2) | 39 (45.9) | 31 (49.2) | 29 (49.2) | 14 (29.2) | ||||
| LSCS | 9 (10.6) | 11 (12.9) | 8 (12.7) | 7 (11.9) | 5 (10.4) | ||||
| Both SVD & LSCS | 8 (9.4) | 4 (4.7) | 5 (7.9) | 3 (5.1) | 4 (8.3) | ||||
| Nil | 33 (38.8) | 31 (36.5) | 19 (32.0) | 20 (33.9) | 25 (52.1) | ||||
| Pre-pregnancy UIŌĆĪ | 0.679 | 0.649 | 0.251 | ||||||
| Yes | 15 (17.6) | 13 (15.3) | 13 (20.6) | 10 (16.9) | 5 (10.4) | ||||
| No | 70 (82.4) | 72 (84.7) | 50 (79.4) | 49 (83.1) | 43 (89.6) | ||||
| History of smokingŌĆĀ | 0.682 | 0.366 | 0.322 | ||||||
| Yes | 4 (4.7) | 2 (2.4) | 4 (6.3) | 1 (1.7) | 1 (2.1) | ||||
| No | 81 (95.3) | 82 (96.5) | 59 (93.7) | 58 (98.3) | 46 (95.8) | ||||
| Currently smoking | - | 1 (1.2) | 0 | 0 | 1 (2.1) | ||||
| Health historyŌĆĀ | |||||||||
| Nil | 59 (69.4) | 57 (67.1) | 0.990 | 43 (68.3) | 41 (69.5) | 0.856 | 32 (66.7) | 0.950 | |
| UTI 3├Ś per year | 2 (2.4) | 3 (3.5) | 2 (3.2) | 2 (3.4) | 1 (2.1) | ||||
| Heart problem | 2 (2.4) | 3 (3.5) | 1 (1.6) | 2 (3.4) | 2 (4.2) | ||||
| Asthma | 7 (8.2) | 8 (9.4) | 4 (6.3) | 6 (10.2) | 5 (10.4) | ||||
| Depression | 1 (1.2) | - | 1 (1.6) | 0 | - | ||||
| Others | 14 (16.5) | 14 (16.5) | 12 (19.0) | 8 (13.6) | 8 (16.7) | ||||
| ConstipationŌĆĪ | 0.153 | 0.233 | 0.827 | ||||||
| Never | 27 (31.8) | 34 (40.0) | 20 (31.7) | 25 (42.4) | 16 (33.3) | ||||
| Seldom (less than 1├Ś monthly) | 31 (36.5) | 27 (31.8) | 23 (36.5) | 19 (32.2) | 16 (33.3) | ||||
| Sometimes (less than 2├Ś weekly) | 17 (20.0) | 21 (24.7) | 12 (19.0) | 13 (22.0) | 13 (27.1) | ||||
| Often (at least 3├Ś weekly) | 10 (11.8) | 3 (3.5) | 8 (12.7) | 2 (3.4) | 3 (6.3) | ||||
| Type of UIŌĆĀ | 0.486 | 0.660 | 0.064 | ||||||
| Nil | 50 (58.8) | 58 (68.2) | 34 (54.0) | 37 (62.7) | 37 (77.1) | ||||
| SUI | 30 (35.3) | 24 (28.2) | 25 (39.7) | 19 (32.2) | 10 (20.8) | ||||
| UUI | 5 (5.9) | 3 (3.5) | 4 (6.3) | 3 (5.1) | 1 (2.1) | ||||
| Continence statusŌĆĪ | 0.200 | 0.328 | 0.022 | ||||||
| Continence | 50 (58.8) | 58 (68.2) | 34 (54.0) | 37 (62.7) | 37 (77.1) | ||||
| Incontinence | 35 (41.2) | 27 (31.8) | 29 (46.0) | 22 (37.3) | 11 (22.9) | ||||
Values are presented as mean┬▒standard deviation or number of respondents (%). P-value significant at P<0.05.
SVD, spontaneous vaginal delivery; LSCS, lower segment cesarean section; UI, urinary incontinence; SUI, stress urinary incontinence; UTI, urinary tract infection; UUI, urge urinary incontinence.
Table┬Ā3.
| Outcome measure and timepoints | IG (n=63) | CG (n=59) | Difference or OR between group (95% CI) | P-value | |
|---|---|---|---|---|---|
| Knowledge | |||||
| ŌĆā | Baseline* | 7.27┬▒1.57 | 7.07┬▒1.62 | 0.20 (-0.95 to 1.35) | 1.000 |
| Early third trimester change from baselineŌĆĀ | -7.29┬▒0.76 | -1.25┬▒0.62 | 6.04 (4.11 to 7.96) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -7.32┬▒0.75 | -1.61┬▒0.65 | 5.71 (3.77 to 7.65) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -8.14┬▒0.69 | -1.56┬▒0.64 | 6.58 (4.74 to 8.43) | <0.001 | |
| Late third trimester change from early third trimesterŌĆĀ | -0.03┬▒0.16 | -0.36┬▒0.27 | -0.32 (-0.93 to 0.28) | 0.295 | |
| Early postnatal change from early third trimesterŌĆĀ | -0.86┬▒0.38 | -0.31┬▒0.29 | 0.55 (-0.39 to1.49) | 0.249 | |
| Early postnatal change from late third trimesterŌĆĀ | -0.83┬▒0.34 | 0.05┬▒0.12 | 0.88 (0.17 to 1.58) | 0.015 | |
| Attitude | |||||
| Baseline* | 30.73┬▒8.83 | 30.45┬▒8.87 | 0.28 (-0.54 to 1.10) | 1.000 | |
| Early third trimester change from baselineŌĆĀ | -3.02┬▒0.91 | 0.58┬▒0.67 | 3.60 (1.37 to 5.82) | 0.002 | |
| Late third trimester change from baselineŌĆĀ | -2.56┬▒0.97 | 0.59┬▒0.59 | 3.15 (0.92 to 5.3) | 0.006 | |
| Early postnatal change from baselineŌĆĀ | -3.43┬▒0.94 | 0.54┬▒0.61 | 3.97 (1.78 to 6.16) | <0.001 | |
| Late third trimester change from early third trimesterŌĆĀ | 0.46┬▒0.55 | 0.02┬▒0.42 | -0.44 (-1.81 to 0.92) | 0.524 | |
| Early postnatal change from early third trimesterŌĆĀ | -0.41┬▒0.66 | -0.03┬▒0.46 | 0.38 (-1.21 to 1.96) | 0.639 | |
| Early postnatal change from late third trimesterŌĆĀ | -0.87┬▒0.36 | -0.05┬▒0.14 | 0.82 (0.06 to 1.58) | 0.034 | |
| Practice | |||||
| Baseline* | 9.07┬▒2.05 | 8.62┬▒2.23 | 0.45 (-0.54 t0 1.46) | 1.000 | |
| Early third trimester change from baselineŌĆĀ | -6.40┬▒0.74 | -1.00┬▒0.50 | 5.40 (3.65 to 7.15) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -7.35┬▒0.78 | -1.42┬▒0.53 | 5.93 (4.08 to 7.78) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -7.97┬▒0.74 | -1.39┬▒0.52 | 6.58 (4.82 to 8.34) | <0.001 | |
| Late third trimester change from early third trimesterŌĆĀ | -0.95┬▒0.43 | -0.42┬▒0.23 | 0.53 (-0.43 to -0.49) | 0.279 | |
| Early postnatal change from early third trimesterŌĆĀ | -1.57┬▒0.51 | -0.39┬▒0.25 | 1.18 (0.07 to 2.30) | 0.038 | |
| Early postnatal change from late third trimesterŌĆĀ | -0.62┬▒0.33 | 0.03┬▒0.08 | 0.65 (0.00 to 1.31) | 0.051 | |
| Self-efficacy | |||||
| Baseline* | 36.50┬▒4.75 | 34.18┬▒4.99 | 2.33 (-13.59 to 18.25) | 1.000 | |
| Early third trimester change from baselineŌĆĀ | -34.53┬▒3.53 | 1.46┬▒2.86 | 35.99 (27.08 to 44.90) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -38.51┬▒3.48 | 0.54┬▒3.16 | 39.05 (29.83 to 48.26) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -40.02┬▒3.25 | 1.91┬▒3.26 | 41.93 (32.91 to 50.96) | <0.001 | |
| Late third trimester change from early third trimesterŌĆĀ | -3.97┬▒2.18 | -0.92┬▒1.88 | 3.06 (-2.59 to 8.70) | 0.289 | |
| Early postnatal change from early third trimesterŌĆĀ | -5.49┬▒2.40 | 0.46┬▒2.03 | 5.94 (-0.22 to 12.11) | 0.059 | |
| Early postnatal change from late third trimesterŌĆĀ | -1.51┬▒1.12 | 1.38┬▒0.77 | 2.89 (0.22 to 5.55) | 0.034 | |
| Severity of urinary incontinence | |||||
| Baseline* | 2.97┬▒0.67 | 2.79┬▒0.72 | 0.18 (-0.38 to 0.74) | 1.000 | |
| Early third trimester change from baselineŌĆĀ | 0.30┬▒0.31 | -0.81┬▒0.37 | -1.12 (-2.07 to -0.16) | 0.022 | |
| Late third trimester change from baselineŌĆĀ | 0.81┬▒0.34 | -1.29┬▒0.39 | -2.10 (-3.11 to -1.09) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | 0.57┬▒0.36 | -1.97┬▒0.45 | -2.54 (-3.67 to -1.41) | <0.001 | |
| Late third trimester change from early third trimesterŌĆĀ | 0.51┬▒0.16 | -0.47┬▒0.21 | -0.98 (-1.50 to 0.47) | <0.001 | |
| Early postnatal change from early third trimesterŌĆĀ | 0.27┬▒0.24 | -1.15┬▒0.31 | -1.42 (-2.19 to -0.66) | <0.001 | |
| Early postnatal change from late third trimesterŌĆĀ | -0.24┬▒0.18 | -0.68┬▒0.25 | -0.44 (-1.04 to 0.16) | <0.001 | |
| Self-reported urinary incontinence | |||||
| BaselineŌĆĪ | 39.7 (25) | 33.9 (20) | 0.508 | ||
| Early third trimester change from baseline┬¦ | 7.9 (5) | 13.6 (8) | 0.83 (0.48 to 1.43) | 0.499 | |
| Late third trimester change from baseline┬¦ | 14.3 (9) | 16.9 (10) | 0.63 (0.33 to 1.18) | 0.149 | |
| Early postnatal change from baseline┬¦ | 22.2 (14) | 27.1 (16) | 0.601 (0.29 to 1.24) | 0.170 | |
| Late third trimester change from early third trimester┬¦ | 6.4 (4) | 3.3 (2) | 0.76 (0.55 to 1.05) | 0.099 | |
| Early postnatal change from early third trimester┬¦ | 14.3 (9) | 13.5 (8) | 0.725 (0.44 to 1.19) | 0.206 | |
| Early postnatal change from late third trimester┬¦ | 7.9 (5) | 10.2 (6) | 0.961 (0.66 to 1.40) | 0.834 | |
Values are presented as estimated mean┬▒standard error or % (number of respondents). P-value significant P<0.05.
IG, intervention group; CG, control group; OR, odds ratio; CI, confidence interval.
ŌĆĀ the changes over time between group (expressed as Exp [╬▓] or exponentiation of the beta coefficient) based on generalized estimating equation model: time, group, and group by time interaction adjusted for baseline value and covariates.
Table┬Ā4.
| Outcome measure and timepoints | IG (n=85) | CG (n=85) | Difference or OR between group (95% CI) | P-value | |
|---|---|---|---|---|---|
| Knowledge | |||||
| ŌĆā | Baseline* | 6.78┬▒0.93 | 6.52┬▒0.96 | -0.26 (-1.19 to 0.68) | 0.590 |
| Early third trimester change from baselineŌĆĀ | -7.27┬▒0.60 | -2.96┬▒0.55 | 4.31 (2.72 to 5.90) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -7.56┬▒0.61 | -3.38┬▒0.58 | 4.18 (2.53 to 5.83) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -6.39┬▒0.67 | -5.80┬▒0.68 | 0.60 (-1.27 to 2.46) | 0.530 | |
| Late third trimester change from early third trimesterŌĆĀ | -0.30┬▒0.16 | -0.43┬▒0.23 | -0.13 (-0.67 to 0.41) | 0.641 | |
| Early postnatal change from early third trimesterŌĆĀ | 0.87┬▒0.43 | -2.84┬▒0.66 | -3.71 (-5.26 to -2.17) | <0.001 | |
| Early postnatal change from late third trimesterŌĆĀ | 1.17┬▒0.43 | -2.41┬▒0.66 | -3.58 (-5.14 to -2.03) | <0.001 | |
| Attitude | |||||
| Baseline* | 30.36┬▒4.75 | 30.12┬▒4.75 | -0.24 (-1.04 to 0.56) | 0.559 | |
| Early third trimester change from baselineŌĆĀ | -2.88┬▒0.76 | -0.14┬▒0.58 | 2.74 (0.87 to 4.62) | 0.004 | |
| Late third trimester change from baselineŌĆĀ | -2.52┬▒0.80 | -0.15┬▒0.55 | 2.37 (0.48 to 4.27) | 0.014 | |
| Early postnatal change from baselineŌĆĀ | -3.71┬▒0.57 | -3.40┬▒0.56 | 0.31 (-1.26 to 1.88) | 0.701 | |
| Late third trimester change from early third trimesterŌĆĀ | 0.36┬▒0.52 | -0.01┬▒0.39 | -0.37 (-1.64 to -0.91) | 0.570 | |
| Early postnatal change from early third trimesterŌĆĀ | -0.82┬▒0.67 | -3.26┬▒0.63 | -2.44 (-4.24 to 0.64) | 0.008 | |
| Early postnatal change from late third trimesterŌĆĀ | -1.18┬▒0.73 | -3.25┬▒0.62 | -2.07 (-3.93 to -0.20) | 0.030 | |
| Practice | |||||
| Baseline* | 8.73┬▒1.16 | 8.33┬▒1.26 | -0.40 (-1.09 to 0.28) | 0.249 | |
| Early third trimester change from baselineŌĆĀ | -6.48┬▒0.56 | -1.83┬▒0.46 | 4.65 (3.24 to 6.07) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -7.46┬▒0.61 | -2.22┬▒0.52 | 5.24 (3.68 to 6.81) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -5.20┬▒0.55 | -5.13┬▒0.51 | 0.07 (-1.41 to 1.55) | 0.928 | |
| Late third trimester change from early third trimesterŌĆĀ | -0.98┬▒0.33 | -0.39┬▒0.27 | 0.59 (-2.24 to 1.43) | 0.164 | |
| Early postnatal change from early third trimesterŌĆĀ | 1.28┬▒0.48 | -3.30┬▒0.49 | -4.58 (-5.93 to -3.24) | <0.001 | |
| Early postnatal change from late third trimesterŌĆĀ | 2.26┬▒0.56 | -2.91┬▒0.62 | -5.18 (-6.81 to -3.68) | <0.001 | |
| Self-efficacy | |||||
| Baseline* | 34.00┬▒3.81 | 32.79┬▒3.77 | -1.22 (-10.14 to 7.71) | 0.789 | |
| Early third trimester change from baselineŌĆĀ | -34.43┬▒3.06 | -5.83┬▒2.91 | 28.60 (20.32 to 36.88) | <0.001 | |
| Late third trimester change from baselineŌĆĀ | -38.91┬▒3.11 | -6.69┬▒3.21 | 32.22 (23.46 to 40.99) | <0.001 | |
| Early postnatal change from baselineŌĆĀ | -18.19┬▒3.29 | 12.98┬▒3.27 | 5.22 (-3.87 to 14.31) | 0.260 | |
| Late third trimester change from early third trimesterŌĆĀ | -4.49┬▒1.55 | -0.86┬▒1.59 | 3.62 (-0.73 to 7.98) | 0.103 | |
| Early postnatal change from early third trimesterŌĆĀ | 16.23┬▒2.09 | -7.15┬▒2.84 | -23.38 (-30.29 to 16.47) | <0.001 | |
| Early postnatal change from late third trimesterŌĆĀ | 20.72┬▒2.34 | -6.29┬▒2.86 | -27.00 (-34.24 to -19.77) | <0.001 | |
| Severity of urinary incontinence | |||||
| Baseline* | 2.73┬▒0.54 | 2.39┬▒0.55 | -0.34 (-0.92 to 0.23) | 0.238 | |
| Early third trimester change from baselineŌĆĀ | -0.13┬▒0.28 | -0.99┬▒0.35 | -0.86 (-1.74 to 0.02) | 0.054 | |
| Late third trimester change from baselineŌĆĀ | 0.17┬▒0.35 | -1.50┬▒0.37 | -1.67 (-2.66 to -0.68) | 0.001 | |
| Early postnatal change from baselineŌĆĀ | -2.00┬▒0.43 | -3.04┬▒0.42 | -1.04 (-2.21 to 0.14) | 0.084 | |
| Late third trimester change from early third trimesterŌĆĀ | 0.30┬▒0.17 | -0.51┬▒0.20 | -0.81 (-1.33 to -0.29) | 0.002 | |
| Early postnatal change from early third trimesterŌĆĀ | -1.87┬▒0.32 | -2.05┬▒0.38 | -0.17 (-1.14 to 0.79) | 0.724 | |
| Early postnatal change from late third trimesterŌĆĀ | -2.18┬▒0.27 | -1.54┬▒0.37 | 0.64 (-0.25 to 1.52) | 0.160 | |
| Self-reported urinary incontinence | |||||
| BaselineŌĆĪ | 41.2 (35) | 31.8 (27) | - | 0.202 | |
| Early third trimester change from baseline┬¦ | 12.9 (11) | 17.6 (15) | 0.78 (0.37 to 1.69) | 0.535 | |
| Late third trimester change from baseline┬¦ | 15.3 (13) | 21.2 (18) | 0.77 (0.29 to 2.07) | 0.604 | |
| Early postnatal change from baseline┬¦ | 15.3 (13) | 10.6 (9) | 1.26 (0.45 to 3.55) | 0.659 | |
| Late third trimester change from early third trimester┬¦ | 2.4 (2) | 3.5 (3) | 0.95 (0.45 to 2.02) | 0.898 | |
| Early postnatal change from early third trimester┬¦ | 2.4 (2) | 7.1 (-6) | 1.59 (0.55 to 4.60) | 0.392 | |
| Early postnatal change from late third trimester┬¦ | 0 | 10.6 (-9) | 1.68 (0.65 to 4.39) | 0.286 | |
Values are presented as estimated mean┬▒standard error or % (number of respondents). P-value significant P<0.05.
IG, intervention group; CG, control group; OR, odd ratio; CI, confidence interval.
ŌĆĀ the changes over time between group (expressed as Exp [╬▓] or exponentiation of the beta coefficient) based on generalized estimating equation model: time, group, and group by time interaction adjusted for baseline value and covariates.
Table┬Ā5.
| Outcome measure and timepoints | IG | CG | Parameters | Estimated mean difference or OR between groups (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Knowledge | ||||||
| ŌĆā | Baseline* (n=170, IG:85, CG:85) | 6.96┬▒1.23 | 6.88┬▒1.25 | Early third trimester change from baselineŌĆĀ | 6.48 (4.53 to 8.43) | <0.001 |
| Early third trimester* (n=112, IG: 58, CG:54) | 14.85┬▒1.30 | 8.29┬▒1.29 | Late third trimester change from baselineŌĆĀ | 5.78 (3.72 to 7.83) | <0.001 | |
| Late third trimester* (n=82, IG:42, CG:40) | 14.65┬▒1.31 | 8.78┬▒1.29 | Early postnatal change from baselineŌĆĀ | 9.02 (6.99 to 11.05) | <0.001 | |
| Early postnatal* (n=21, IG:10, CG:11) | 17.18┬▒1.23 | 8.07┬▒1.29 | Late third trimester change from early third trimesterŌĆĀ | -0.88 (-2.09 to 0.34) | 0.156 | |
| Early postnatal change from early third trimesterŌĆĀ | 1.72 (-1.30 to 4.75) | 0.264 | ||||
| Early postnatal change from late third trimesterŌĆĀ | 2.52 (-0.65 to 5.69) | 0.119 | ||||
| Attitude | ||||||
| Baseline* (n=170, IG:85, CG:85) | 30.48┬▒6.49 | 30.41┬▒6.50 | Early third trimester change from baselineŌĆĀ | 3.92 (1.53 to 6.30) | 0.001 | |
| Early third trimester* (n=112, IG: 58, CG:54) | 33.85┬▒6.46 | 29.85┬▒6.50 | Late third trimester change from baselineŌĆĀ | 2.65 (0.06 to 5.24) | 0.045 | |
| Late third trimester* (n=82, IG:42, CG:40) | 32.56┬▒6.40 | 29.84┬▒6.53 | Early postnatal change from baselineŌĆĀ | 6.59 (3.81 to 9.38) | <0.001 | |
| Early postnatal* (n=21, IG:10, CG:11) | 37.28┬▒6.38 | 30.61┬▒6.55 | Late third trimester change from early third trimesterŌĆĀ | -2.10 (-4.24 to 0.03) | 0.054 | |
| Early postnatal change from early third trimesterŌĆĀ | 1.21 (-2.32 to 4.73) | 0.503 | ||||
| Early postnatal change from late third trimesterŌĆĀ | 3.27 (-0.41 to 6.95) | 0.082 | ||||
| Practice | ||||||
| Baseline* (n=170, IG:85, CG:85) | 8.82┬▒1.74 | 8.64┬▒1.81 | Early third trimester change from baselineŌĆĀ | 5.96 (4.18 to 7.73) | <0.001 | |
| Early third trimester* (n=112, IG: 58, CG:54) | 15.85┬▒1.86 | 9.71┬▒1.77 | Late third trimester change from baselineŌĆĀ | 6.56 (4.49 to 8.64) | <0.001 | |
| Late third trimester* (n=82, IG:42, CG:40) | 16.91┬▒1.95 | 10.17┬▒1.83 | Early postnatal change from baselineŌĆĀ | 8.10 (5.83 to 10.38) | <0.001 | |
| Early postnatal* (n=21, IG:10, CG:11) | 17.76┬▒2.07 | 9.48┬▒1.82 | Late third trimester change from early third trimesterŌĆĀ | 0.43 (-1.10 to -1.97) | 0.579 | |
| Early postnatal change from early third trimesterŌĆĀ | 2.38 (-0.58 to 5.34) | 0.115 | ||||
| Early postnatal change from late third trimesterŌĆĀ | 1.96 (-1.31 to 5.23) | 0.239 | ||||
| Self-efficacy | ||||||
| Baseline* (n=170, IG:85, CG:85) | 34.07┬▒4.51 | 32.93┬▒4.36 | Early third trimester change from baselineŌĆĀ | 45.86 (35.82 to 55.90) | <0.001 | |
| Early third trimester* (n=112, IG: 58, CG:54) | 73.08┬▒3.32 | 32.55┬▒4.53 | Late third trimester change from baselineŌĆĀ | 46.66 (34.86 to 58.47) | <0.001 | |
| Late third trimester* (n=82, IG:42, CG:40) | 79.38┬▒3.84 | 32.38┬▒4.79 | Early postnatal change from baselineŌĆĀ | 4.89 (-2.35 to 12.12) | <0.001 | |
| Early postnatal* (n=21, IG:10, CG:11) | 73.47┬▒4.24 | 25.67┬▒5.45 | Late third trimester change from early third trimesterŌĆĀ | 39.39 (30.64 to 48.12) | 0.189 | |
| Early postnatal change from early third trimesterŌĆĀ | 7.17 (-3.90 to 18.23) | 0.204 | ||||
| Early postnatal change from late third trimesterŌĆĀ | 6.38 (-6.47 to 19.23) | 0.330 | ||||
| Severity of urinary incontinence | ||||||
| Baseline* (n=170, IG:85, CG:85) | 2.83┬▒0.60 | 2.69┬▒0.62 | Early third trimester change from baselineŌĆĀ | -1.16 (-2.07 to -0.15) | 0.024 | |
| Early third trimester* (n=112, IG: 58, CG:54) | 2.56┬▒0.62 | 3.59┬▒0.73 | Late third trimester change from baselineŌĆĀ | -2.40 (-3.58 to -1.23) | <0.001 | |
| Late third trimester* (n=82, IG:42, CG:40) | 1.98┬▒0.66 | 4.25┬▒0.72 | Early postnatal change from baselineŌĆĀ | -3.81 (-6.36 to -1.26) | 0.003 | |
| Early postnatal* (n=21, IG:10, CG:11) | 3.57┬▒1.10 | 7.24┬▒1.10 | Late third trimester change from early third trimesterŌĆĀ | -1.28 (-2.15 to -0.41) | 0.004 | |
| Early postnatal change from early third trimesterŌĆĀ | -2.14 (-4.88 to -0.60) | 0.126 | ||||
| Early postnatal change from late third trimesterŌĆĀ | -0.89 (-3.69 to 1.91) | 0.532 | ||||
| Self-reported urinary incontinence | ||||||
| BaselineŌĆĪ (n=170, IG:85, CG:85) | 41.2 (35) | 31.8 (27) | Early third trimester change from baseline┬¦ | 0.81 (0.46 to 1.44) | 0.481 | |
| Early third trimesterŌĆĪ (n=112, IG: 58, CG:54) | 55.2 (32) | 48.1 (26) | Late third trimester change from baseline┬¦ | 0.54 (0.26 to 1.10) | 0.090 | |
| Late third trimesterŌĆĪ (n=82, IG:42, CG:40) | 52.4 (22) | 57.5 (23) | Early postnatal change from baseline┬¦ | 0.54 (0.07 to 4.13) | 0.555 | |
| Early postnatalŌĆĪ (n=21, IG:10, CG:11) | 70 (7) | 81.8 (9) | Late third trimester change from early third trimester┬¦ | 0.74 (0.42 to 1.30) | 0.297 | |
| Early postnatal change from early third trimester┬¦ | 0.67 (0.10 to 4.48) | 0.679 | ||||
| Early postnatal change from late third trimester┬¦ | 1.04 (0.13 to 8.53) | 0.973 | ||||
Values are presented as estimated mean┬▒standard error or % (number of respondents). P-value significant P<0.05.
IG, intervention group; CG, control group; OR, odd ratio; CI, confidence interval.
ŌĆĀ the changes over time between group (expressed as Exp [╬▓] or exponentiation of the beta coefficient) based on generalized estimating equation model: time, group, and group by time interaction adjusted for baseline value and covariates.







