|
 |
- Search
| Korean J Fam Med > Volume 35(2); 2014 > Article |
Abstract
Background
Abnormal serum gamma-glutamyltransferase (γ-GT) may be an early and sensitive marker for oxidative stress. This study was performed to evaluate the association between serum heavy metals and γ-GT concentration.
Methods
This study is a cross-sectional analysis based on data from Korean National Health and Nutrition Examination Survey (V-1, 2, 2010, 2011) regarding serum heavy metal concentrations (lead, mercury, and cadmium) as well as serum γ-GT. Serum heavy metals were categorized into tertiles, and serum γ-GT concentration was compared using an analysis of covariance test after relevant variable adjustments. In addition, we evaluated the odds ratio (OR) of having the highest tertile of serum γ-GT in each heavy metal tertile using logistic regression.
Results
The mean serum lead, mercury, and cadmium concentrations were 2.67, 5.08, and 1.02 µg/dL in men and 1.95, 3.60, and 1.21 µg/dL in women, respectively. Partial correlation showed a significant positive relation between each heavy metal and serum γ-GT concentration. Comparing serum γ-GT concentration by the tertile of each heavy metal, serum γ-GT concentration showed a significant increase as the tertiles of serum mercury and cadmium in men and that of serum mercury in women increased, but not with lead. The OR of having the highest tertile of serum γ-GT was significant for cadmium in men (OR, 4.02; 95% confidence interval [CI], 2.54 to 6.35) and mercury in women (OR, 2.00; 95% CI, 1.29 to 3.10) in the top tertile of each heavy metal.
Exposure to heavy metals is common in modern life. Heavy metals include both those that are essential for normal biological functions (e.g., Cu and Zn), and those which are non-essential, such as cadmium, mercury, and lead. It is known that both essential and non-essential metals may disturb normal biological functions. One of the major mechanisms of heavy metal toxicity has been attributed to oxidative stress, which is supported by the wide spectrum of nucleobase products typical for the oxygen attack on DNA in cultured cells and animals.1) Heavy metals are environmental chemicals that are detected by biomonitoring. The term environmental chemical refers to a chemical compound or chemical element present in air, water, food, soil, dust, or other environmental media. Biomonitoring is the assessment of human exposure to chemicals by measuring the chemicals or their metabolites in human specimens such as blood or urine.2)
Elevated serum gamma-glutamyltransferase (γ-GT) might be an early and sensitive marker for oxidative stress.3) There is evidence that cellular γ-GT plays an important role in antioxidant defense systems.4) γ-GT is widely distributed in the human body and is frequently localized in the plasma membrane with its active site directed into the extracellular space. In addition, the primary role of γ-GT is to metabolize extracellular reduced glutathione (GSH), allowing for precursor amino acids to be assimilated and reutilized for intracellular GSH synthesis. Thus, ectoplasmic γ-GT acts as a cellular supply of GSH, the most important non-protein antioxidant of the cell.5) According to a recent study, ectoplasmic γ-GT may also be involved in the generation of harmful metal-induced reactive oxygen species.6) In addition, in many animal studies, metal-induced oxidative stress can affect not only biomonitoring data but also biological lab data, including γ-GT.7) Nonetheless, there have not been many human studies on γ-GT elevation and biomonitoring data due to heavy metal exposure. The aim of the present study is to evaluate the association of heavy metal concentrations (lead, mercury, and cadmium) and serum γ-GT concentration using the fifth Korean National Health and Nutrition Examination Survey (KNHANES) data (V-1, 2, 2010, 2011).
The KNHANES has been conducted periodically by the Korea Centers for Disease Control and Prevention since 1998 and provides comprehensive information on health status, health behavior, nutritional status, and sociodemographics in 600 national districts in Korea. KNHANES data (V-1, 2, 2010, 2011) samplings containing serum heavy metal concentrations (lead, mercury, and cadmium) including serum γ-GT data were used in this cross-sectional analysis. From an initial total of 17,476 men and women, 12,926 people were excluded due to missing data (the missing data of serum γ-GT, 3,959 subjects; heavy metal data missing, 8,767 subjects). Two hundred and twenty-four subjects who had medical illness were also excluded (liver diseases, cancer, and rheumatoid disease). We defined liver disease as viral hepatitis B, liver cirrhosis, and hepatitis B carriers. Those younger subjects than 20 years old (799 subjects) were also excluded. After that, 200 subjects whose serum γ-GT was above 200 IU/L were further excluded. In total, 3,677 subjects (1,806 men and 1,876 women) were included in this analysis.
Serum γ-GT concentration was assayed by the standard method recommended by the International Federation for Clinical Chemistry using pure auto γ-GT as substrate with a Hitachi Automatic Analyzer 7600 (Hitachi Co., Tokyo, Japan). Lead was assayed by standard methods using DMA-8 as a substrate with HNO3 (coefficient of variation [CV]% range was 1.0 to 26.7 µg/dL). Mercury and cadmium were checked by standard methods using a Perkin Elmer AAnalyst 60 (Perkin-Elmer, Norwalk, CT, USA) with the atomic absorption method using ammonium phosphate and TritonX-10 as substrates. The CV% ranges of serum mercury and cadmium were 0.7 to 38.9 µg/L and 0.3 to 10.1 µg/L, respectively. Blood pressure was measured using a standard mercury manometer and expressed by rounding off. Fasting blood specimens were used for measuring lipids, glucose, and liver enzymes. We used a self-administered questionnaire of hypertension, diabetes, smoking status, job, education, moderate physical activity, and alcohol intake for proper adjustments. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2).
Complex sample analysis was used for KNHANES data (V-1, 2, 2010, 2011) for weighting all values following the guidance on statistics from the Korea Centers for Disease Control and Prevention. Initially, we used a simple descriptive analysis for general characteristics such as age, BMI, lipid profiles, fasting glucose, blood pressure, and serum heavy metals, including lead, mercury, and cadmium in both genders. Partial correlation analysis was performed to evaluate the association between heavy metal concentration and serum γ-GT concentration in both genders after adjusting for age, BMI, smoking, and alcohol intake. To evaluate the exact relation between serum heavy metals and serum γ-GT concentration, we categorized serum heavy metals into tertiles and compared serum γ-GT concentration using an analysis of covariance test after adjustments for age, BMI, job, education, moderate physical activity, smoking status, and alcohol intake in men, and menopause, oral contraceptive use, and hormone replacement therapy in women. Finally, we calculated the odds ratio (OR) of having the highest tertile of serum γ-GT in the tertiles of serum mercury and cadmium in men, and that of mercury in women using logistic regression analysis after adjustments for age, BMI, job, education, moderate physical activity, smoking status, and alcohol intake in men, and menopause, oral contraceptive use, and hormone replacement therapy in women. The P-value for trends was used to assess the significance of all analysis, and P < 0.05 was considered significant. Data were analyzed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
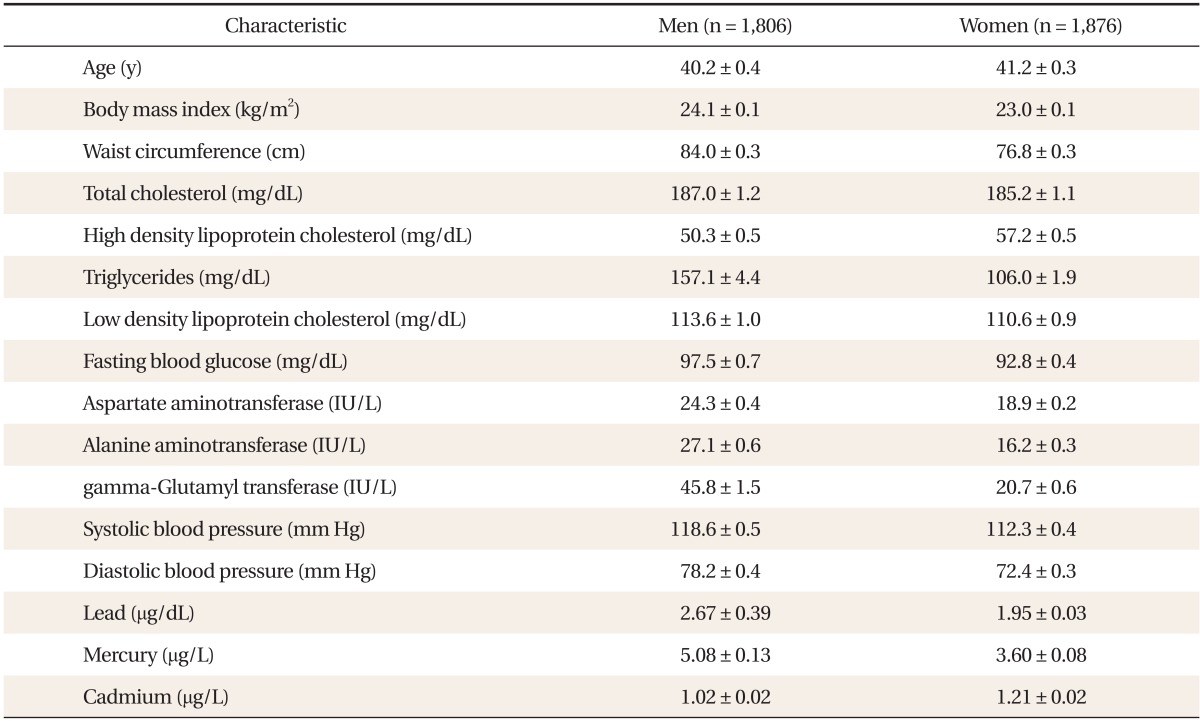
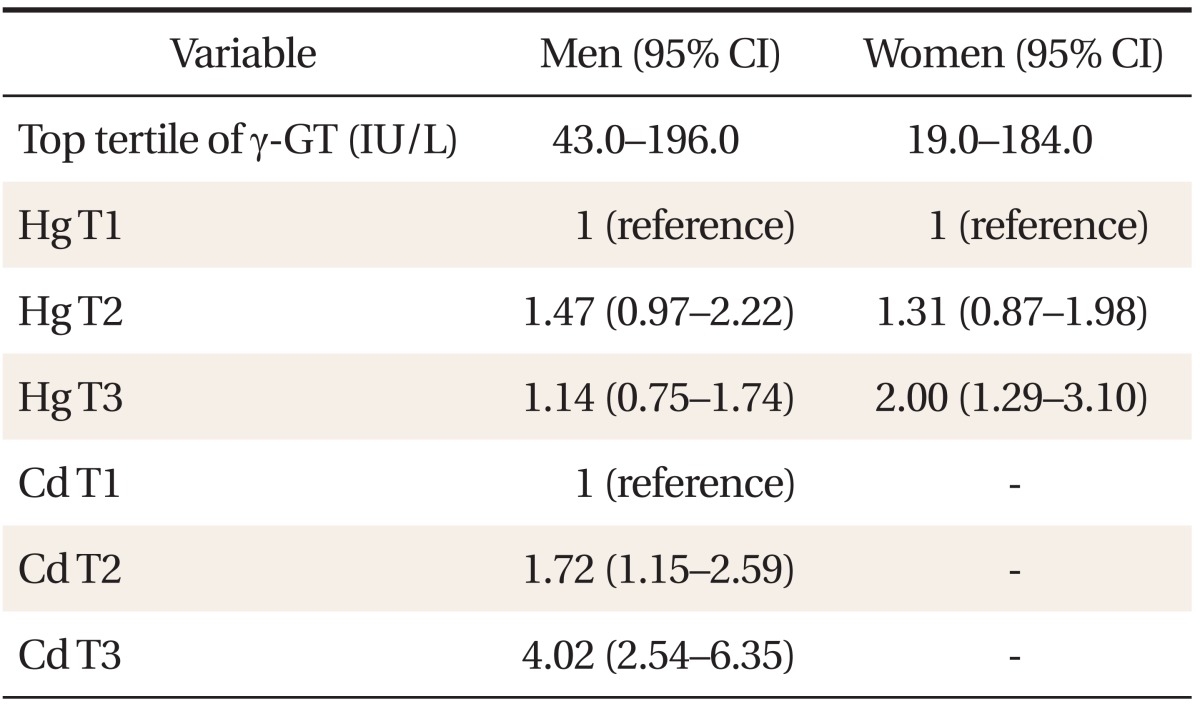
Table 1 shows the general characteristics of the study subjects. Compared with females, males showed higher serum concentration of lead, mercury, and γ-GT. In contrast, serum cadmium concentration was higher in women than in men. Table 2 shows a partial correlation between heavy metal concentration and γ-GT after adjusting for age, BMI, smoking status, and alcohol intake. In men, lead (r = 0.064, P < 0.05), mercury (r = 0.134, P < 0.001), and cadmium (r = 0.243, P < 0.001) showed significant correlations with serum γ-GT concentration. In women, lead (r = 0.032, P = 0.017), mercury (r = 0.096, P < 0.001), and cadmium (r = 0.112, P < 0.001) also showed significance. Table 3 shows γ-GT concentrations according to increasing heavy metal tertiles. With the increasing tertile of mercury in men and women, serum γ-GT concentration was significantly higher in the second and third tertiles than in the first tertile. In particular, serum γ-GT concentration showed the highest mean value in the top tertile compared with other tertiles of serum cadmium in men (P < 0.001). When comparing the OR of having the highest tertile of serum γ-GT, the adjusted OR in the 2nd and top tertile of serum cadmium in men was 1.72 (95% confidence interval [CI], 1.15 to 2.59), 4.02 (95% CI, 2.54 to 6.35), and the adjusted OR of the top tertile of mercury in women was 2.00 (95% CI, 1.29 to 3.10). The adjusted OR of mercury in men was not statistically significant.
The present study demonstrated that data from KNHANES (V-1, 2, 2010, 2011) showed that the serum concentration of cadmium in men and that of mercury in women has a significant relation with serum γ-GT concentration. In addition, the overall correlation of serum heavy metal with serum γ-GT concentration was considerable in both genders.
There have not been many human studies evaluating the relationship between serum heavy metal and γ-GT concentration. In a lead exposure study, values of liver function test (alkaline phosphatase [P = 0.012] and lactate dehydrogenase [P = 0.029]) were significantly higher in industrial workers who had significantly higher mean blood lead concentration (77.5 ± 42.8 mg/dL) than non-industrial workers (19.8 ± 12.3 mg/dL). These results might be related to lead exposure, and liver function might have been affected.8) In an animal study, tissue analysis showed an accumulation of lead, cadmium, and mercury in the liver and kidney of brown hares, but there was no significant correlation between levels of heavy metals in the liver and kidney and biochemical parameters.9)
Traditionally, serum γ-GT activity has been used as a marker for excessive alcohol consumption or liver diseases in clinical practice. A dose response relationship is generally seen in which the mean γ-GT increases as the amount of self-reported alcohol consumption increases.10) Heavy metal intoxication may result in oxidative stress. Some studies suggest that serum γ-GT concentration within its normal laboratory range might be an early and sensitive marker for oxidative stress.3) Therefore, serum γ-GT elevation after drinking alcohol might be interpreted as a reflection of oxidative stress; acute and chronic ethanol consumption under experimental conditions increased the production of reactive oxygen species.11)
In our study results, cadmium in men and mercury in women showed significantly higher ORs of having the highest tertile of serum γ-GT concentration. Unfortunately, because we could not differentiate the quantitative measurements of heavy metal exposure in both genders, it is hard to conclude this difference between genders or direct connection between serum heavy metals and γ-GT concentration. In a cadmium toxicity study, cadmium caused oxidative stress, mainly because it has a great affinity for thiols, especially GSH, which is the most important antioxidant in aerobic organisms.12) Some studies show serum γ-GT activity increased in response to cadmium loading, which may mean serum γ-GT and glutathione work together to remove cadmium from the cytoplasm, a crucial mechanism for metal detoxification that is dependent on glutathione.13) Epidemiologic data showed that mercury poisoning mainly resulted from longterm occupational exposure to mercury, abuse of mercury-containing compounds, or skin-lightening cosmetics.14) Among mercury exposure routes, amalgam-related mercury exposure exceeds that from fish for the majority of the population.15) In a recent study, mercury poisoning was commonly related to mercury-containing skin-lightening cream or mercury-containing anti-rheumatoid pills.16) In our study results, although serum mercury concentration in women was not higher than in men, their serum mercury accumulation might be from chronic cream and pill usage, which may have been related with serum γ-GT elevation.
In cadmium exposure, diet is the main source of environmental cadmium exposure. Tobacco smoking results in 4 to 7 times higher blood cadmium and two to three times higher kidney cadmium concentrations in smokers than in nonsmokers.17,18) The concentrations of cadmium in blood, urine, and kidneys were found to have been generally higher in women than in men.19,20) This can be explained by the mechanism of uptake for iron and cadmium, the duodenal metal transporter, which is known to be upregulated by iron deficiency and to have high affinity for cadmium.21) In our study, however, serum γ-GT concentration showed the highest mean adjusted value and OR in the top tertile of serum cadmium, even after the adjustment for serum iron as well as adjusting for smoking history and alcohol intake, which may mean serum cadmium concentration is an independent factor with regard to higher serum γ-GT concentration. Considering that serum γ-GT together with glutathione is supposed to work to remove cadmium or mercury from the body, and abnormal γ-GT concentration may indicate oxidative stress partially from serum heavy metals, higher serum concentration of cadmium in men and mercury in women may therefore be related with higher serum γ-GT concentration.
This study has several limitations. First, urine mercury concentration is generally considered a better marker than serum samples, but there is no such data in KNHANES data. Second, we could not adjust for all heavy metal exposure routes or confounders such as the amount of tobacco smoking, alcohol intake, fish consumption, and occupational exposure to heavy metals. Third, we used a cross-sectional design, so we cannot draw causality from these results. The strength of this study is the relatively large scale including both genders, and that data were representative of the general population of Korea. In addition, this is the first study to deal with serum heavy metals and serum γ-GT concentration in the Korean population.
In conclusion, the elevation of serum heavy metal may be related to the elevation of serum γ-GT concentration. In particular, serum cadmium in men and mercury in women showed significant correlation with serum γ-GT concentration. It is necessary to investigate whether decreasing serum heavy metals decreases or normalizes serum γ-GT concentration in the near future.
References
1. Chen F, Ding M, Castranova V, Shi X. Carcinogenic metals and NF-kappaB activation. Mol Cell Biochem 2001;222:159-171. PMID: 11678598.


2. Centers for Disease Control and Prevention. Fact sheet: the fourth national report on human exposure to environmental chemicals, 2009 (US) [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2009. [cited 2013 Aug 13]. Available from: http://www.cdc.gov/exposurereport
3. Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res 2004;38:535-539. PMID: 15346644.


4. Karp DR, Shimooku K, Lipsky PE. Expression of gamma-glutamyl transpeptidase protects ramos B cells from oxidation-induced cell death. J Biol Chem 2001;276:3798-3804. PMID: 11080500.


5. Forman HJ, Liu RM, Tian L. Glutathione cycling in oxidative stress. Lung Biol Health Dis 1997;105:99-122.
6. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12:1161-1208. PMID: 15892631.


7. Maellaro E, Dominici S, Del Bello B, Valentini MA, Pieri L, Perego P, et al. Membrane gamma-glutamyl transpeptidase activity of melanoma cells: effects on cellular H(2)O(2) production, cell surface protein thiol oxidation and NF-kappa B activation status. J Cell Sci 2000;113(Pt 15):2671-2678. PMID: 10893182.



8. Al-Neamy FR, Almehdi AM, Alwash R, Pasha MA, Ibrahim A, Bener A. Occupational lead exposure and amino acid profiles and liver function tests in industrial workers. Int J Environ Health Res 2001;11:181-188. PMID: 11382350.


9. Kolesarova A, Slamecka J, Jurcik R, Tataruch F, Lukac N, Kovacik J, et al. Environmental levels of cadmium, lead and mercury in brown hares and their relation to blood metabolic parameters. J Environ Sci Health A Tox Hazard Subst Environ Eng 2008;43:646-650. PMID: 18393073.


10. Teschke R, Brand A, Strohmeyer G. Induction of hepatic microsomal gamma-glutamyltransferase activity following chronic alcohol consumption. Biochem Biophys Res Commun 1977;75:718-724. PMID: 16594.


11. Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med 2001;31:1524-1526. PMID: 11744324.


12. Penninckx MJ. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res 2002;2:295-305. PMID: 12702279.


13. Adamis PD, Mannarino SC, Eleutherio EC. Glutathione and gamma-glutamyl transferases are involved in the formation of cadmium-glutathione complex. FEBS Lett 2009;583:1489-1492. PMID: 19345220.


14. Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, et al. Mercury poisoning associated with a Mexican beauty cream. West J Med 2000;173:15-18. PMID: 10903281.



15. Richardson GM, Wilson R, Allard D, Purtill C, Douma S, Graviere J. Mercury exposure and risks from dental amalgam in the US population, post-2000. Sci Total Environ 2011;409:4257-4268. PMID: 21782213.


16. Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, et al. Mercury-induced membranous nephropathy: clinical and pathological features. Clin J Am Soc Nephrol 2010;5:439-444. PMID: 20089494.



17. Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 2009;238:201-208. PMID: 19409405.


18. Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 2010;23:769-782. PMID: 20517707.


19. Alfven T, Elinder CG, Carlsson MD, Grubb A, Hellstrom L, Persson B, et al. Low-level cadmium exposure and osteoporosis. J Bone Miner Res 2000;15:1579-1586. PMID: 10934657.


20. Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health 1998;24(Suppl 1):1-51. PMID: 9569444.

21. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388:482-488. PMID: 9242408.


Table 2
Partial correlation of serum Pb, Hg, Cd concentration with serum gamma-glutamyltransferase concentration
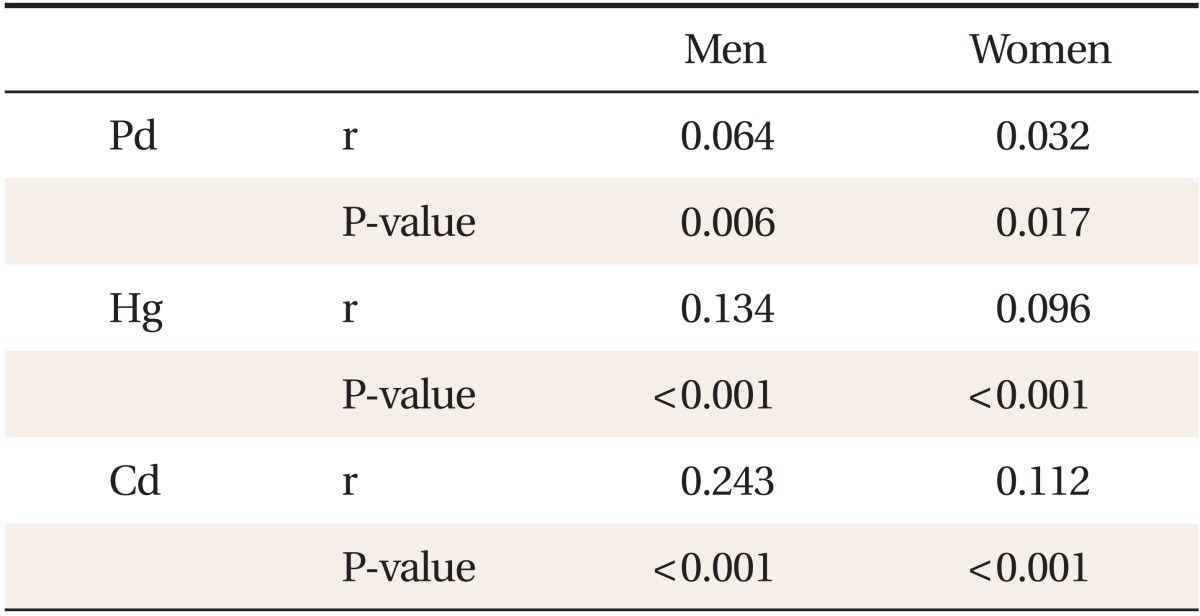
Table 3
serum γ-GT concentration by heavy metal tertiles
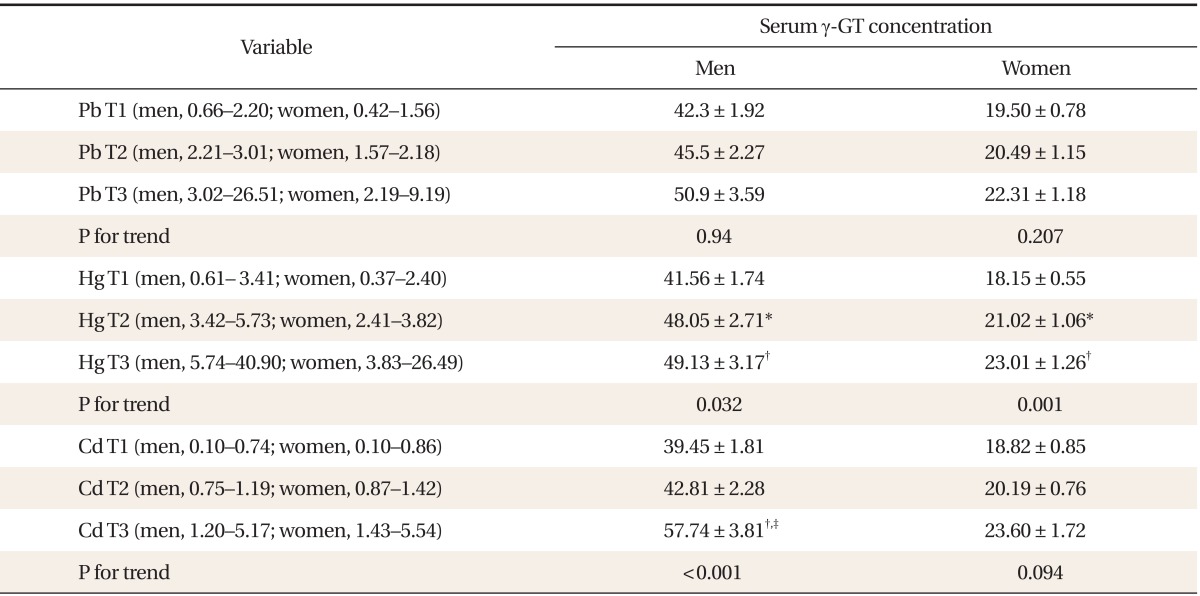
Values are presented as mean ± standard error. P-for trend are obtained by analysis of covariance test after adjustment for age, body mass index, job, education , moderate physical activity, smoking status, alcohol intake, and serum iron concentration. T1-T3 represent each tertile of heavy metal. ( ) represent range in each tertile of heavy metal.
γ-GT: gamma-glutamyltransferase.
*P < 0.05 in the comparison of T1 vs. T2. †P < 0.05 in the comparison of T1 vs. T3. ‡P < 0.05 in the comparison of T2 vs. T3.
- TOOLS