|
 |
- Search
| Korean J Fam Med > Volume 44(2); 2023 > Article |
|
Abstract
With aging, loss of skeletal muscle mass and muscle function increases, resulting in an increased risk of falls, fractures, long-term institutional care, cardiovascular and metabolic diseases, and even death. Sarcopenia, which is derived from the Greek words “sarx” or flesh+“penia” or loss, is defined as a condition characterized by low muscle mass and low muscle strength and performance. In 2019, the Asian Working Group for Sarcopenia (AWGS) published a consensus paper on the diagnosis and treatment of sarcopenia. The AWGS 2019 guideline, specifically, presented strategies for case-finding and assessment to help diagnose “possible sarcopenia” in primary care settings. For case finding, the AWGS 2019 guideline proposed an algorithm that recommends calf circumference measurement (cut-off <34 cm in men, <33 cm in women) or the SARC-F (strength, assistance with walking, rising from a chair, climbing stairs, and falls) questionnaire (cut-off ≥4). If this case finding is confirmed, handgrip strength (cutoff <28 kg in men, <18 kg in women) or the 5-time chair stand test (≥12 seconds) should be performed to diagnose “possible sarcopenia.” If an individual is diagnosed as “possible sarcopenia,” AWGS 2019 recommends that the individual should start lifestyle interventions and related health education for primary healthcare users. Because no medication is available to treat sarcopenia, exercise and nutrition is essential for sarcopenia management. Many guidelines, recommend physical activity, with a focus on progressive resistance (strength) training, as a first-line therapy for the management of sarcopenia. It is essential to educate older adults with sarcopenia on the need to increase protein intake. Many guidelines recommended that older people should consume at least 1.2 g of proteins/kg/d. This minimum threshold can be increased in the presence of catabolic or muscle wasting. Previous studies reported that leucine, a branched-chain amino acid, is essential for protein synthesis in muscle, and a stimulator for skeletal muscle synthesis. A guideline conditionally recommends that diet or nutritional supplements should be combined with exercise intervention for older adults with sarcopenia.
With aging, loss of skeletal muscle mass and muscle function increases, which results in an increased risk of falls, fractures, long-term institutional care, cardiovascular and metabolic diseases, and even death [1]. Sarcopenia, which is derived from the Greek words “sarx” or flesh+“penia” or loss, has been defined as a condition characterized by low skeletal muscle mass together with low muscle strength and low muscle performance with aging [1]. In 2019, the Asian Working Group for Sarcopenia (AWGS 2019) published a consensus paper on the diagnosis and treatment of sarcopenia. The AWGS 2019 guidelines, specifically recommended strategies for case-finding and assessment to help diagnose “possible sarcopenia” in primary care settings [1].
Globally, sarcopenia is estimated to be between 8.4% and 27.6% in community-dwelling older persons, 14%–33% in long-term care residents, and 10% in the acute hospital care population [2]. In Korea, the prevalence of sarcopenia in community-dwelling older adults (70–84 years), defined as low muscle mass plus low handgrip strength and slow gait speed according to the AWGS 2019 guideline, was 21.3% in men and 13.8% in women from a nationwide Korean Frailty and Aging Cohort Study [3]. Sarcopenia is more likely to be present in men than in women and tends to increase with advancing age [2]. Asians with a low body mass index were at a higher risk of sarcopenia [2].
Primary care physicians need to be familiar with sarcopenia management for two reasons [4]: (1) Sarcopenia is considered a reversible condition that can be improved with nutritional counseling and personalized physical activity programs. (2) Management of sarcopenia can be effective when the primary care physician, who is the most familiar with the clinical characteristics and behaviors of the patient, plays an active role.
Low muscle mass, muscle strength, and/or physical performance are required to diagnose sarcopenia [1]. The AWGS 2019 guideline recommends using either dual-energy X-ray absorptiometry (DXA) or bioimpedance analysis (BIA), though magnetic resonance imaging and computed tomography are more accurate skeletal muscle mass measurement [1]. The AWGS 2019 cutoffs for low muscle mass in sarcopenia diagnosis are as follows: <7.0 kg/m2 in men and <5.4 kg/m2 in women by DXA; and <7.0 kg/m2 in men and <5.7 kg/m2 in women by BIA. The AWGS 2019 recommends low muscle strength diagnostic cutoffs of handgrip <28.0 kg for men and <18.0 kg for women.
As to physical performance, AWGS 2019 recommends assessment of low physical performance based on either short physical performance battery, 6-m walk, or 5-time chair stand test [1]. The cutoff of usual gait speed for 6-m walk test in AWGS 2019 guideline is 1.0 m/s. The 5-time chair stand time was proposed as a surrogate for gait speed in sarcopenia diagnosis, and ≥12 seconds was recommended as the cutoff for low physical performance by AWGS 2019.
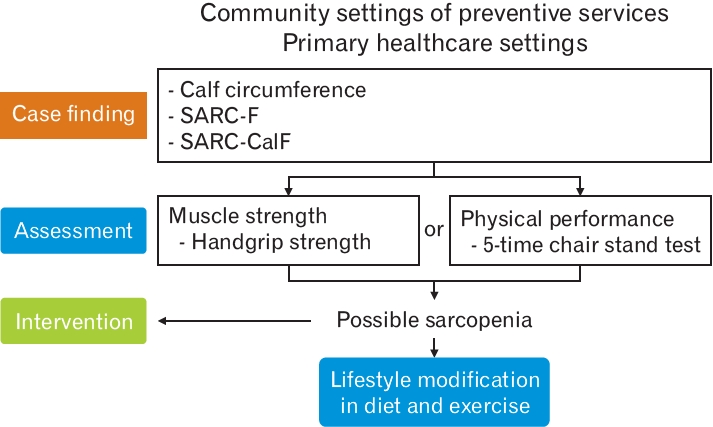
Considering the difficulty of measuring muscle mass in community settings, AWGS 2019 suggested the term “possible sarcopenia” for early identification of older adults with or at risk for sarcopenia to facilitate necessary interventions in primary care settings without advanced diagnostic equipment [1]. For case-finding in “possible sarcopenia,” AWGS 2019 recommends using either calf circumference (CC) or the SARC-F (strength, assistance with walking, rising from a chair, climbing stairs, and falls) questionnaire. In predicting sarcopenia or low skeletal muscle mass, the recommended measurement of CC with the maximum value of both calves using a nonelastic tape has moderate to high sensitivity and specificity in predicting sarcopenia or low skeletal muscle mass [1]. For screening or case finding, AWGS 2019 recommends CC <34 cm for men and <33 cm for women. The SARC-F questionnaire assesses five components: strength, assistance in walking, rising from a chair, climbing stairs, and falling. The cutoff of the SARC-F score was 4 out of a total score of 10. In Korea, the author’s group translated the SARC-F questionnaire into the Korean language in a culturally responsive way and validated it [5]. The SARC-F ≥4 group was well associated with poorer grip strength, slower walking speed, and poorer physical performance [5].
However, the presence of any of the following clinical conditions can replace case-finding: functional decline or limitation, unintentional weight loss, depressive mood, cognitive impairment, repeated falls, malnutrition, and chronic conditions (heart failure, chronic obstructive pulmonary disease, diabetes mellitus, chronic kidney disease, etc.) [1]. Similarly, a recent consensus paper promoting the identification and management of sarcopenia in primary care proposed the so-called “Red Flag Method” including clinician’s observation, patient’s presenting features, and clinician’s assessment [6].
- Clinician’s observation: weakness, visual identification of low muscle mass, slow gait speed
- Patient’s presenting features: loss of weight, loss of muscle strength in arms or in legs, general weakness, fatigue, falls, mobility impairment, loss of energy, difficulties in physical activities or activities of daily living
- Clinician’s assessment: malnutrition, low body weight, low physical activity
If the case finding is confirmed, AWGS 2019 recommends checking handgrip strength measurement (cut-off <28 kg in men, <18 kg in women) or the 5-time chair stand test (cut-off ≥12 seconds), and “possible sarcopenia” is diagnosed based on either result (Figure 1).
Participants with “possible sarcopenia” seems to differ from those with “sarcopenia” in some characteristics [7]. When authors defined “possible sarcopenia” as a combination of using the low CC or the SARC-F questionnaire and 5-time chair stand, without involving handgrip strength based on the AWGS 2019 guidelines, women were more likely to have “possible sarcopenia”, while men were more likely to have sarcopenia. Those with possible sarcopenia had a lower educational level, lower functional ability, lower quality of life, and more cognitive dysfunction than those with sarcopenia [7].
The AWGS 2019 recommends that primary healthcare users with “possible sarcopenia” begin lifestyle interventions and related health education regardless of the final diagnosis of sarcopenia. However, they are also encouraged to visit their referral hospital if a confirmatory diagnosis is needed.
The author group surveyed primary care physicians based in Seoul under a study funded by the National Evidence-based Healthcare Collaborating Agency. While more than 90% of the physicians agreed that early diagnosis of sarcopenia is vital, only 51.2% of the doctors were willing to test for sarcopenia at their clinics. However, 48.8% of those who did not want to perform the tests reported that they would test for sarcopenia if the handgrip test is excluded for diagnosing “possible sarcopenia” (unpublished data).
Because no medication is available to treat sarcopenia, exercise and nutrition are essential for sarcopenia management. Primary care physicians are ideally situated to incorporate the concept of sarcopenia into their practice, as they are champions of comprehensive care, including education on nutrition and exercise [8].
Primary care physicians may provide recommendations for managing reversible risk factors (e.g., sedentary behavior, unhealthy diet) and eventually refer them to specialists for further evaluation if needed [2].
Physical activity, with a focus on progressive resistance (strength) training, is recommended by guidelines as first-line therapy for sarcopenia [9]. Resistance-based training refers to any physical activity which produces skeletal muscle contractions by using external resistance such as dumbbells, free weights, elastic therapy bands and body weight itself [9]. The health benefits of resistance-based training for older adults include muscle hypertrophy, strength gain, and improved physical performance [9].
In addition to resistance training programs, multicomponent exercise interventions, including robust resistance training, have been shown to improve not only muscle strength but also risk of falls in older adults with frailty and sarcopenia [10]. In general, multicomponent or combined exercise programs, including aerobic activities, resistance training, and flexibility exercises, are recommended [11].
Programs consisting of home-based exercise interventions, weight-bearing exercises, or very low workloads are much less effective for achieving strength gains [10] or treating sarcopenia than higher-intensity prescriptions [11]. Nevertheless, a 6-month home exercise program that combined walking and resistance lower-limb exercises (squats, single-leg standing, and heel raise) were effective in improving maximum walking speed and muscle strength in individuals ≥60 years old with low muscle mass or sarcopenia [12].
Cruz-Jentoft et al. [13] proposed two recommendations regarding the management of physical activity/exercise interventions in older people. First, the duration of the intervention should be at least 3 months to obtain an impact on muscle function. Second, supervised resistance exercises or multi-component or combined exercise programs should be recommended for frail or sedentary individuals who live in community.
Education to improve protein intake in older adults with sarcopenia is important. The nutritionist review highlighted that balanced dietary patterns should be provided in addition to protein intake. That is, healthy fat/omega-3 and hydration is important, with the quality of calories ingested (processed versus non-processed foods) and the impact of medications on nutritional intake [9].
Many guidelines recommended the assumption of at least 1.2 g of proteins/kg/d in older persons, pushing even higher this minimum threshold in the presence of catabolic or muscle wasting conditions [14-16]. In Korea, the current recommended daily allowance for protein intake is 0.91 g/kg/d for the general older adult population, which is just for maintaining the current muscle mass, and more amount of protein intake is needed to increase muscle mass in sarcopenic adults [16].
Studies have suggested that some amino acids related to dietary protein may affect anabolic synthesis in skeletal muscle. Previous studies have suggested that leucine, a branched-chain amino acid, is essential for protein synthesis in muscle and also as a stimulator of skeletal muscle synthesis [16]. In fact, leucine can increase muscle protein synthesis in older people, as confirmed in a recent meta-analysis [2].
Guidelines conditionally recommend that diet or nutritional supplementation should be combined with a physical activity intervention for older adults with sarcopenia [9]. Although the fractional muscle protein synthesis rate in older men was 16% lower than that in young men following ingestion of 20 g protein, the difference disappeared if they intake protein along with exercise [17].
Combined nutritional-physical activity intervention can improve gait speed and knee extension strength compared to individual physical activity or nutritional intervention [9]. Resistance exercise coupled with leucine-enriched essential amino acid supplements or whey protein food supplements (when the diet is inadequate in terms of energy and protein) is recommended to treat sarcopenia [18].
Patients with sarcopenia with low vitamin D levels (<20 ng/mL measured by a 25-hydroxyvitamin D test) are recommended to receive vitamin D supplements [9]. A meta-analysis reported that the effects on muscle strength increase are prominent in older people presenting a baseline 25[OH]D concentration lower than 30 nmol/L (12 ng/mL) and in older people institutionalized or hospitalized [19].
In 2019, the AWGS guideline published an algorithm for diagnosis of “possible sarcopenia” in primary care settings. If diagnosed as “possible sarcopenia,” AWGS 2019 recommends initiation of lifestyle interventions and related health education for primary healthcare users, because no medication is available to treat sarcopenia, and exercise and nutrition are essential for sarcopenia management. Physical activity, with a focus on progressive resistance (strength) training, is recommended by many guidelines as first-line therapy to manage sarcopenia. Education to improve protein intake in older adults with sarcopenia is also important. Many guidelines recommend the assumption of at least 1.2 g of proteins/kg/d in older persons, pushing this minimum threshold even higher in the presence of catabolic or muscle wasting. Previous studies have suggested that leucine, a branched-chain amino acid, is essential for protein synthesis in muscle and as a stimulator for skeletal muscle synthesis. It is frequently recommended that diet or nutritional supplements should be combined with exercise interventions for older adults with sarcopenia.
REFERENCES
1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300-7.


2. Crosignani S, Sedini C, Calvani R, Marzetti E, Cesari M. Sarcopenia in primary care: screening, diagnosis, management. J Frailty Aging 2021;10:226-32.

3. Kim M, Won CW. Sarcopenia in Korean community-dwelling adults aged 70 years and older: application of screening and diagnostic tools from the Asian Working Group for Sarcopenia 2019 update. J Am Med Dir Assoc 2020;21:752-8.


4. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817-33.




5. Kim S, Kim M, Won CW. Validation of the Korean version of the SARCF Questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 2018;19:40-5.


6. Beaudart C, McCloskey E, Bruyere O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016;16:170.




7. Shin HE, Kim M, Won CW. Differences in characteristics between older adults meeting criteria for sarcopenia and possible sarcopenia: from research to primary care. Int J Environ Res Public Health 2022;19:4312.



8. Won CW. Diagnosis and management of frailty in primary health care. Korean J Fam Med 2020;41:207-13.




9. Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging 2018;22:1148-61.



10. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians: effects on skeletal muscle. JAMA 1990;263:3029-34.


11. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging 2021;25:824-53.



12. Maruya K, Asakawa Y, Ishibashi H, Fujita H, Arai T, Yamaguchi H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J Phys Ther Sci 2016;28:3183-8.



13. Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review: report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748-59.



14. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929-36.



15. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542-59.


16. Jung HW, Kim SW, Kim IY, Lim JY, Park HS, Song W, et al. Protein intake recommendation for Korean older adults to prevent sarcopenia: expert consensus by the Korean Geriatric Society and the Korean Nutrition Society. Ann Geriatr Med Res 2018;22:167-75.



17. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322-31.


- TOOLS
-
METRICS

- Related articles in KJFM
-
Diagnosis and Management of Frailty in Primary Health Care2020 July;41(4)
Avascular Necrosis of the Hip in Primary Care2021 January;42(1)
Overview of Physical Activity Counseling in Primary Care2021 July;42(4)
Religion and Health Behaviors in Primary Care Patients2020 March;41(2)